Metabolic activation of 3-hydroxyanisole by isolated rat hepatocytes.
Majid Y Moridani, Sophia S Cheon, Sumsullah Khan, Peter J O'Brien
文献索引:Chem. Biol. Interact. 142(3) , 317-33, (2003)
全文:HTML全文
摘要
A tyrosinase-directed therapeutic approach for malignant melanoma therapy uses the depigmenting phenolic agents such as 4-hydroxyanisole (4-HA) to form cytotoxic o-quinones. However, renal and hepatic toxicity was reported as side effects in a recent 4-HA clinical trial. In search of novel therapeutics, the cytotoxicity of the isomers 4-HA, 3-HA and 2-HA were investigated. In the following, the order of the HAs induced hepatotoxicity in mice, as measured by increased in vivo plasma transaminase activity, or in isolated rat hepatocytes, as measured by trypan blue exclusion, was 3-HA > 2-HA > 4-HA. Hepatocyte GSH depletion preceded HA induced cytotoxicity and a 4-MC-SG conjugate was identified by LC/MS/MS mass spectrometry analysis when 3-HA was incubated with NADPH/microsomes/GSH. 3-HA induced hepatocyte GSH depletion or GSH depletion when 3-HA was incubated with NADPH/microsomes was prevented by CYP 2E1 inhibitors. Dicumarol (an NAD(P)H: quinone oxidoreductase inhibitor) potentiated 3-HA- or 4-methoxycatechol (4-MC) induced toxicity whereas sorbitol (an NADH generating nutrient) greatly prevented cytotoxicity indicating a quinone-mediated cytotoxic mechanism. Ethylendiamine (an o-quinone trap) largely prevented 3-HA and 4-MC-induced cytotoxicity indicating that o-quinone was involved in cytotoxicity. Dithiothreitol (DTT) greatly reduced 3-HA and 4-MC induced toxicity. The ferric chelator deferoxamine slightly decreased 3-HA and 4-MC induced cytotoxicity whereas the antioxidants pyrogallol or TEMPOL greatly prevented the toxicity suggesting that oxidative stress contributed to 3-HA induced cytotoxicity. In summary, ring hydroxylation but not O-demethylation/epoxidation seems to be the bioactivation pathway for 3-HA in rat liver. The cytotoxic mechanism for 3-HA and its metabolite 4-MC likely consists cellular protein alkylation and oxidative stress. These results suggest that 3-HA is not suitable for treatment of melanoma.Copyright 2002 Elsevier Science B.V.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
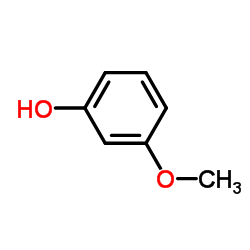 |
3-甲氧基苯酚
CAS:150-19-6 |
C7H8O2 |
|
Convenient QSAR model for predicting the complexation of str...
2009-01-01 [Bioorg. Med. Chem. 17 , 896-904, (2009)] |
|
Calculating virtual log P in the alkane/water system (log P(...
2005-05-05 [J. Med. Chem. 48 , 3269-79, (2005)] |
|
Cellular apoptosis and cytotoxicity of phenolic compounds: a...
2005-11-17 [J. Med. Chem. 48 , 7234-42, (2005)] |
|
Action mechanism of tyrosinase on meta- and para-hydroxylate...
2000-04-01 [Biol. Chem. 381(4) , 313-20, (2000)] |
|
Isolation and characterization of a veratrol:corrinoid prote...
2001-05-01 [Arch. Microbiol. 175(5) , 376-83, (2001)] |