| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
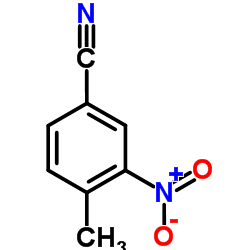 |
4-甲基-3-硝基氰苯
CAS:939-79-7 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
4-甲基-3-硝基氰苯
CAS:939-79-7 |