Allergic contact dermatitis to topical preparations of bufexamac.
Yan Pan, Rosemary Nixon
文献索引:Australas. J. Dermatol. 53(3) , 207-10, (2012)
全文:HTML全文
摘要
In Australia bufexamac is mainly used for pharmacist-initiated local treatment of various dermatoses. The European Medicines Agency's Committee for Medicinal Products for Human Use recently recommended that marketing authorisation for bufexamac-containing preparations be revoked throughout the European Union because of the risk of severe allergic contact dermatitis. We retrospectively reviewed the patch test database at the Skin and Cancer Foundation Inc. and identified 19 cases of positive reactions to bufexamac (5% petrolatum) from 451 people patch tested. The bufexamac reaction was deemed relevant to the presenting dermatitis in 13 of 19 (68%) patients. Bufexamac allergic contact dermatitis is under-reported in the English literature. We wish to emphasise the severity and the unusually polymorphic eruptions observed in some of the cases. Clinicians should consider the possibility of allergic contact dermatitis to bufexamac-containing preparations in all patients where there is a history of exposure, even if used for only a short time.© 2012 The Authors. Australasian Journal of Dermatology © 2012 The Australasian College of Dermatologists.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
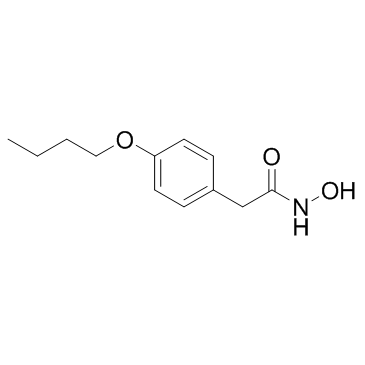 |
丁苯羟酸
CAS:2438-72-4 |
C12H17NO3 |
|
Erythema-multiforme-like, urticarial papular and plaque erup...
2015-02-11 [Contact Dermatitis 31(2) , 97-101, (1994)] |
|
[A severe epicutaneous test reaction to the bufexamac in a h...
1999-10-08 [Dtsch. Med. Wochenschr. 124(40) , 1168-70, (1999)] |
|
[A common and insidious side-effect: allergic contact dermat...
2005-12-16 [Dtsch. Med. Wochenschr. 130(50) , 2881-6, (2005)] |
|
In vitro photobiochemical characterization of sulfobutylethe...
2011-06-01 [J. Pharm. Biomed. Anal. 55(3) , 591-6, (2011)] |
|
Examinations of the antioxidative properties of the topicall...
2003-10-01 [J. Pharm. Pharmacol. 55(10) , 1379-88, (2003)] |