| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
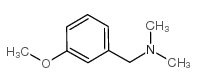 |
3-甲氧基-N,N-二甲基苄胺
CAS:15184-99-3 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
3-甲氧基-N,N-二甲基苄胺
CAS:15184-99-3 |