Organic Letters
2006-01-19
An efficient route to 4-aryl-5-pyrimidinylimidazoles via sequential functionalization of 2,4-dichloropyrimidine.
Xiaohu Deng, Neelakandha S Mani
文献索引:Org. Lett. 8(2) , 269-72, (2006)
全文:HTML全文
摘要
[reaction: see text] Starting from 2,4-dichloropyrimidine, a concise synthetic route to medicinally important 4-aryl-5-pyrimidinylimidazoles is described. Sequential substitution of the 4- and 2-chloro groups using a regioselective Sonogashira coupling, followed by nucleophilic substitution, led to pyrimidinylalkyne derivatives, which were then oxidized to their corresponding 1,2-diketones. These 1,2-diketones, on cyclocondensation with ammonium acetate and an aldehyde, furnished the desired pyrimidinyl imidazoles in good overall yields.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
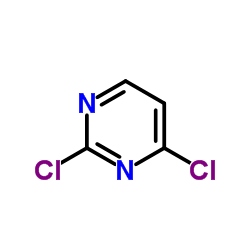 |
2,4-二氯嘧啶
CAS:3934-20-1 |
C4H2Cl2N2 |
相关文献:
更多...
|
Novel vanilloid receptor-1 antagonists: 1. Conformationally ...
2007-07-26 [J. Med. Chem. 50 , 3497, (2007)] |
|
One-pot Double Suzuki Couplings of Dichloropyrimidines.
2012-02-01 [Synthesis 2010(16) , 2721-2724, (2010)] |
|
Chemistry-based risk assessment for skin sensitization: quan...
2011-07-18 [Chem. Res. Toxicol. 24(7) , 1003-11, (2011)] |
|
Selective iron-catalyzed cross-coupling reactions of grignar...
2004-05-28 [J. Org. Chem. 69 , 3943-3949, (2004)] |
|
Synthesis and SAR of aminopyrimidines as novel c-Jun N-termi...
2007-06-15 [Bioorg. Med. Chem. Lett. 17 , 3463, (2007)] |