Quantitative analysis of the mixed activating effects of the alkali metal ions on intestinal brush-border sucrase at pH 5.2.
F Alvarado, A Mahmood, C Tellier, M Vasseur
文献索引:Biochim. Biophys. Acta 613(1) , 140-52, (1980)
全文:HTML全文
摘要
The activation of rabbit brush-border sucrase by the alkali metal ions, Li+, Na+ and K+, was analyzed using the equations of the random-order allosteric model previously proposed for sucrase (Mahmood, A. and Alvarado, F. (1975) Arch. Biochem. Biophys. 168, 585). The alkali metals have mixed activating effects in tert-butylamine buffers at pH 5.2, including: 1. Affinity-type activation, where the apparent Km decreases as a hyperbolic function of the metal concentration. 2. Capacity-type activation, where the apparent V increases with the metal concentration. These two effects were analyzed quantitatively: firstly, by using linear transformations that allowed us to solve each partial equation separately and secondly, by iteration of the general equation, which permits treating the mixed effects as a whole. Results are consistent with the interpretation that a single metal-binding (activator) site suffices to explain the simultaneous occurrence of the two types of kinetic effect. Nevertheless, complicating factors exist that may require the postulation of additional sites for monovalent cations. In particular, the tert-butylammonium ion appears to interface with the effects of the alkali metals, especially Li+.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
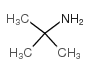 |
叔丁胺
CAS:75-64-9 |
C4H11N |
|
Multilayered Thin Films from Boronic Acid-Functional Poly(am...
2015-09-01 [Pharm. Res. 32(9) , 3066-86, (2015)] |
|
Ion chromatography of amylamine and tert.-butylamine in phar...
1996-07-19 [J. Chromatogr. A. 739(1-2) , 343-9, (1996)] |
|
[Hygienic standard for tert-butylamine in water].
1985-12-01 [Gig. Sanit. (12) , 70-1, (1985)] |
|
Towards molecular diversity: dealkylation of tert-butyl amin...
2010-08-21 [Org. Biomol. Chem. 8(16) , 3631-4, (2010)] |
|
Dynamics of an [Fe4S4(SPh)4]2- cluster explored via IR, Rama...
2006-05-14 [Dalton Trans. (18) , 2192-201, (2006)] |