A practical synthesis of N α-Fmoc protected L-threo-β-hydroxyaspartic acid derivatives for coupling via α- or β-carboxylic group.
Nina Bionda, Maré Cudic, Lidija Barisic, Maciej Stawikowski, Roma Stawikowska, Diego Binetti, Predrag Cudic
文献索引:Amino Acids 42(1) , 285-93, (2012)
全文:HTML全文
摘要
A simple and practical general synthetic protocol towards orthogonally protected tHyAsp derivatives fully compatible with Fmoc solid-phase peptide synthetic methodology is reported. Our approach includes enantioresolution of commercially available D: ,L: -tHyAsp racemic mixture by co-crystallization with L: -Lys, followed by ion exchange chromatography yielding enantiomerically pure L: -tHyAsp and D: -tHyAsp, and their selective orthogonal protection. In this way N ( α )-Fmoc protected tHyAsp derivatives were prepared ready for couplings via either α- or β-carboxylic group onto the resins or the growing peptide chain. In addition, coupling of tHyAsp via β-carboxylic group onto amino resins allows preparation of peptides containing tHyAsn sequences, further increasing the synthetic utility of prepared tHyAsp derivatives.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
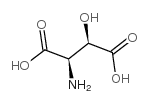 |
DL-苏-beta-羟基天冬氨酸
CAS:4294-45-5 |
C4H7NO5 |
|
The SLC1 high-affinity glutamate and neutral amino acid tran...
2013-01-01 [Mol. Aspects Med. 34(2-3) , 108-20, (2013)] |
|
A concise, asymmetric synthesis of (2R,3R)-3-hydroxyaspartic...
2008-08-01 [Amino Acids 35(2) , 507-10, (2008)] |
|
Insights into the mechanism of Pseudomonas dacunhae aspartat...
2010-06-22 [Biochemistry 49 , 5066-73, (2010)] |
|
Neuroprotective effects of yokukansan, a traditional Japanes...
2009-04-10 [Neuroscience 159(4) , 1397-407, (2009)] |
|
Serine racemase homologue of Saccharomyces cerevisiae has L-...
2003-08-29 [FEMS Microbiol. Lett. 225(2) , 189-93, (2003)] |