| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
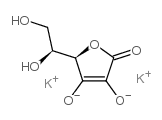 |
L-抗坏血酸-2-硫酸二钾
CAS:52174-99-9 |
|
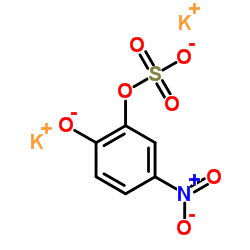 |
4-硝基儿茶酚硫酸二钾盐水合物
CAS:14528-64-4 |