Neurotensin-induced Cl(-) current in guinea-pig dorsal root ganglion cells.
S Kawarada, T Unno, H Ohashi, S Komori
文献索引:Eur. J. Pharmacol. 404(1-2) , 69-78, (2000)
全文:HTML全文
摘要
In guinea-pig dorsal root ganglion cells held under voltage-clamp at -80 mV, neurotensin elicited an inward current (I(NT)) whose amplitude increased with increasing neurotensin concentration (40-4000 nM). The effect was blocked by a nonpeptide neurotensin antagonist. I(NT) occurred in the absence of the extracellular Na(+), but not in the absence of the intracellular Cl(-), and it was outward directed by reversing the driving force for Cl(-). I(NT), like the gamma-amino-butyric acid (GABA)-induced Cl(-) current (I(GABA)), remained little changed after virtual elimination of cytosolic free-ionized Ca(2+) or after treatment with a Ca(2+)-activated Cl(-) channel blocker, but, in contrast to I(GABA) it was resistant to the I(GABA) blocker picrotoxin, slower in time course and more easily desensitized when repeatedly elicited. I(NT) and I(GABA) were additive to each other. AG-protein inhibitor markedly reduced I(NT), and a G-protein activator produced an inward current during which no current could be elicited by neurotensin. These results show that neurotensin exerts an effect to activate Ca(2+)-insensitive Cl(-) channels distinct from those activated by GABA in guinea-pig dorsal root ganglion cells, and the effect may arise through a G-protein-dependent mechanism.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
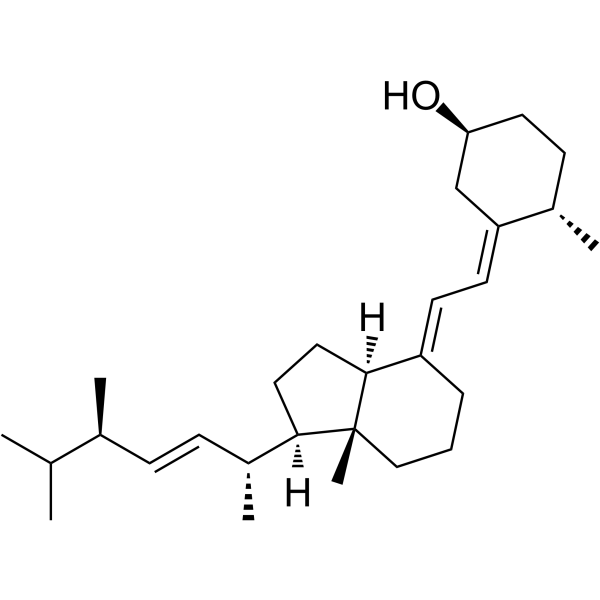 |
多沙唑嗪杂质F
CAS:67-96-9 |
C28H46O |
|
Prevalence and correction of 25(OH) vitamin D deficiency in ...
2005-01-01 [Perit. Dial. Int. 25(4) , 362-6, (2005)] |
|
3-nitropropionic acid-induced hydrogen peroxide, mitochondri...
2005-02-18 [Brain Res. Mol. Brain Res. 133(2) , 215-23, (2005)] |
|
Calpain-mediated AQP2 proteolysis in inner medullary collect...
2003-03-28 [Biochem. Biophys. Res. Commun. 303(1) , 52-8, (2003)] |
|
Functional modulation of the ATP-sensitive potassium channel...
2008-03-18 [Neuroscience 152(2) , 371-80, (2008)] |
|
Early apoptotic and late necrotic components associated with...
2008-05-01 [J. Neurosci. Res. 80(4) , 549-61, (2005)] |