| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
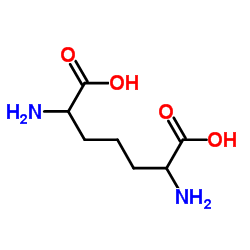 |
2,6-二氨基庚二酸
CAS:583-93-7 |
|
 |
酰胺酶 来源于绿脓假单胞菌
CAS:9012-56-0 |