Thermal isomerization of (+)-cis- and (-)-trans-pinane leading to (-)-beta-citronellene and (+)-isocitronellene.
Achim Stolle, Bernd Ondruschka, Werner Bonrath, Thomas Netscher, Matthias Findeisen, Markus M Hoffmann
文献索引:Chemistry 14(22) , 6805-14, (2008)
全文:HTML全文
摘要
Catalyzed and uncatalyzed rearrangement reactions of terpenoids play a major role in laboratory and industrial-scale synthesis of fine chemicals. Herein, we present our results on the thermally induced isomerization of pinane (1). Investigation of the thermal behavior of (+)-cis- (1 a) and (-)-trans-pinane (1 b) in a flow-type reactor reveals significant differences in both reactivity and selectivity concerning the formation of (-)-beta-citronellene (2) and (+)-isocitronellene (3) as main products. Possible explanations for these results are discussed on the basis of reaction mechanism and ground-state geometries for 1 a and 1 b. To identify side reactions caused from ene cyclizations of 2 and 3, additional pyrolysis experiments were conducted that enabled the identification of almost all compounds in the network of C(10)H(18)-hydrocarbon products formed from 1.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
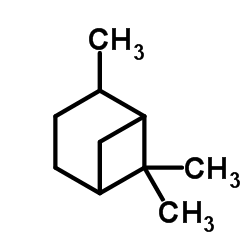 |
(1R)-(+)-CIS 蒎烷
CAS:4795-86-2 |
C10H18 |
|
Metabolic profiling of GuanXin II prescription based on meta...
2011-03-25 [J. Pharm. Biomed. Anal. 54(4) , 789-98, (2011)] |
|
Chemistry around pinene and pinane: a facile synthesis of cy...
2008-06-01 [Chem. Biodivers. 5(6) , 910-9, (2008)] |
|
Acute toxicities to larval rainbow trout of representative c...
1991-02-01 [Bull. Environ. Contam. Toxicol. 46(2) , 173-8, (1991)] |
|
Role of thromboxane and angiotensin in cyclosporine-induced ...
1993-01-01 [J. Heart Lung Transplant. 12(5) , 851-5, (1993)] |
|
Selective boron-containing thrombin inhibitors--X-ray analys...
2000-09-01 [Bioorg. Med. Chem. 8(9) , 2291-303, (2000)] |