| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
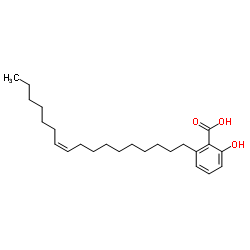 |
银杏酸C17:1
CAS:111047-30-4 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
银杏酸C17:1
CAS:111047-30-4 |