The ortho backbone amide linker (o-BAL) is an easily prepared and highly acid-labile handle for solid-phase synthesis.
Ulrik Boas, Jesper Brask, Jørn B Christensen, Knud J Jensen
文献索引:J. Comb. Chem. 4(3) , 223-8, (2002)
全文:HTML全文
摘要
The tris(alkoxy)benzyl backbone amide linker (BAL) has found widespread application in solid-phase synthesis. The key intermediate for preparation of para BAL (p-BAL) is 2,6-dimethoxy-4-hydroxybenzaldehyde; several reports on its synthesis have appeared. However, the ortho analogue of the handle (o-BAL) has successfully been used by us for the synthesis of C-terminal-modified peptides, oligosaccharides, and substituted anilines. Here, we present a new and convenient synthesis of the key intermediate for o-BAL, 4,6-dimethoxy-2-hydroxybenzaldehyde, by a highly regioselective demethylation with BBr3, followed by purification through steam distillation. Cleavage studies of Leu-enkephalin anchored to either o-BAL or p-BAL handles revealed that both handles were surprisingly acid-labile and released the peptide with dilute TFA (5% and even 1% TFA in CH2Cl2). This useful property allowed the synthesis of fully protected Leu-enkephalin. The very convenient synthesis of 4,6-dimethoxy-2-hydroxybenzaldehyde combined with the benign properties of the o-BAL handle may make it the preferred regioisomer.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
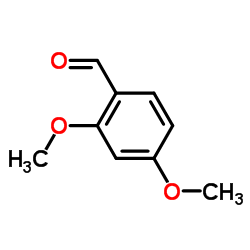 |
2,4-二甲氧基苯甲醛
CAS:613-45-6 |
C9H10O3 |
|
Antifungal activity of redox-active benzaldehydes that targe...
2011-01-01 [Ann. Clin. Microbiol. Antimicrob. 10 , 23, (2011)] |
|
Rational design of inhibitors of VirA-VirG two-component sig...
2007-06-15 [Bioorg. Med. Chem. Lett. 17 , 3281-6, (2007)] |
|
Can phlorotannins purified extracts constitute a novel pharm...
2012-01-01 [PLoS ONE 7(2) , e31145, (2012)] |
|
Anti-proliferative activity and chemical characterization by...
2016-01-08 [J. Chromatogr. A. 1428 , 115-25, (2016)] |
|
Enhancement of solubility by temporary dimethoxybenzyl-subst...
1991-06-01 [Int. J. Pept. Protein Res. 37(6) , 556-64, (1991)] |