| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
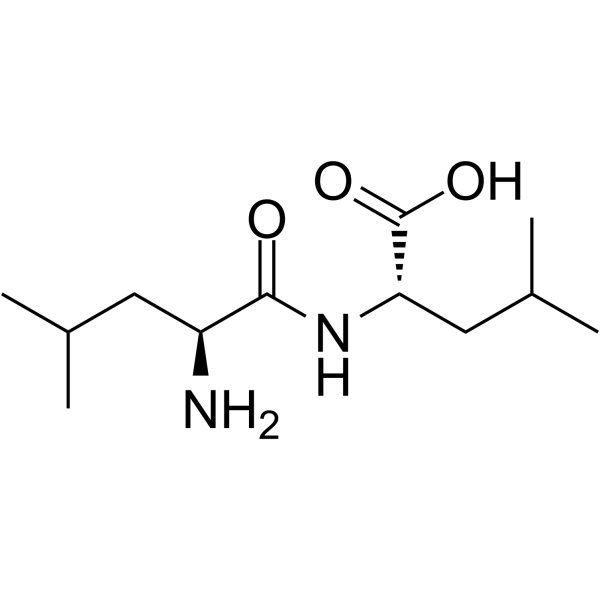 |
(S)-2-((S)-2-氨基-4-甲基戊酰胺基)-4-甲基戊酸
CAS:3303-31-9 |
|
 |
L-亮氨酰-D-亮氨酸二水合物
CAS:17665-02-0 |