| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
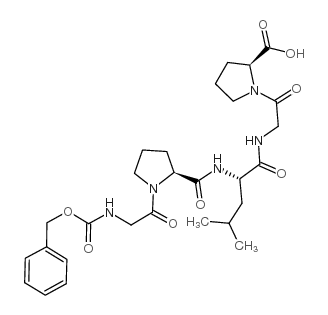 |
Z-Gly-Pro-Leu-Gly-Pro-OH
CAS:2646-61-9 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
Z-Gly-Pro-Leu-Gly-Pro-OH
CAS:2646-61-9 |