[Spectrophotometric determination of iron using eriochrome cyanine R as extractant with phase-separation].
Z Zhang, Y Xie, Y Shi
文献索引:Guang Pu Xue Yu Guang Pu Fen Xi 19(2) , 241-3, (1999)
全文:HTML全文
摘要
Spectrophotometric determination of iron by using eriochrome cyanine R as extractant and color-developing agent in organic solvents was studied. The complex was extracted by PEG phase from the water solutions of pH4.5-6.5 (NaAc-HAc). The maximum absorption of the complex is at 560 nm and the molar absorptivity is 5.36 x 10(4) L x mol(-1) x cm(-1). Beers law was obeyed in the range of 0-40 microg Fe. Iron and eriochrome cyanine R formed a stable complex with a molar ratio of 1:2. The proposed method has been applied to the determination of copper in aluminium alloys with satisfactory results.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
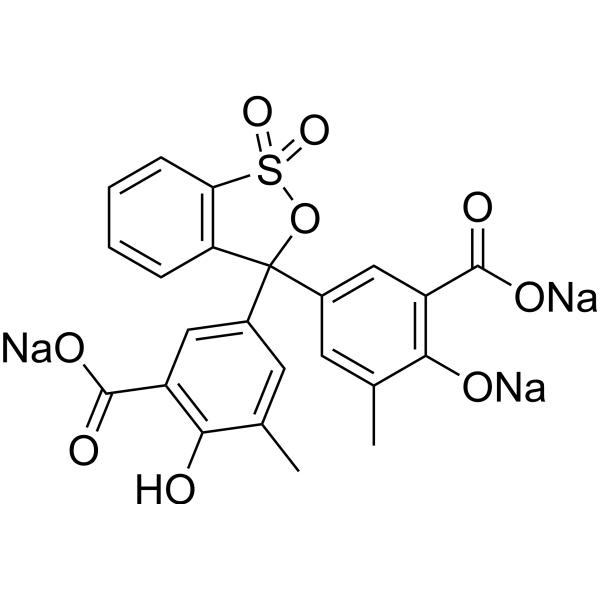 |
依来铬氰蓝R
CAS:3564-18-9 |
C23H15Na3O9S |
|
Transplantation of human neural stem cells transduced with O...
2009-01-01 [BMC Neurosci. 10 , 117, (2009)] |
|
Analytical application of the reactions of amitriptyline wit...
2003-01-01 [Acta Pol. Pharm. 60(6) , 417-23, (2003)] |
|
Chromoxane cyanine R. I. Physical and chemical properties of...
1984-04-01 [J. Microsc. 134(Pt 1) , 13-23, (1984)] |
|
Histological study of the human temporo-mandibular joint and...
2004-10-01 [Surg. Radiol. Anat. 26(5) , 371-8, (2004)] |
|
[Resonance light scattering study on interaction of solochro...
2004-02-01 [Guang Pu Xue Yu Guang Pu Fen Xi 24(2) , 194-6, (2004)] |