Structural basis of carotenoid cleavage: from bacteria to mammals.
Xuewu Sui, Philip D Kiser, Johannes von Lintig, Krzysztof Palczewski
文献索引:Arch. Biochem. Biophys. 539(2) , 203-13, (2013)
全文:HTML全文
摘要
Carotenoids and their metabolic derivatives serve critical functions in both prokaryotic and eukaryotic cells, including pigmentation, photoprotection and photosynthesis as well as cell signaling. These organic compounds are also important for visual function in vertebrate and non-vertebrate organisms. Enzymatic transformations of carotenoids to various apocarotenoid products are catalyzed by a family of evolutionarily conserved, non-heme iron-containing enzymes named carotenoid cleavage oxygenases (CCOs). Studies have revealed that CCOs are critically involved in carotenoid homeostasis and essential for the health of organisms including humans. These enzymes typically display a high degree of regio- and stereo-selectivity, acting on specific positions of the polyene backbone located in their substrates. By oxidatively cleaving and/or isomerizing specific double bonds, CCOs generate a variety of apocarotenoid isomer products. Recent structural studies have helped illuminate the mechanisms by which CCOs mobilize their lipophilic substrates from biological membranes to perform their characteristic double bond cleavage and/or isomerization reactions. In this review, we aim to integrate structural and biochemical information about CCOs to provide insights into their catalytic mechanisms.Copyright © 2013 Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
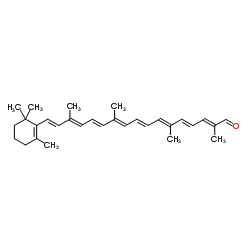 |
全反式-β-阿林胡萝卜醛
CAS:1107-26-2 |
C30H40O |
|
The formation, occurrence, and function of β-apocarotenoids:...
2012-11-01 [Am. J. Clin. Nutr. 96(5) , 1189S-92S, (2012)] |
|
beta-Apo-8'-carotenal, but not beta-carotene, is a strong in...
1996-09-01 [Xenobiotica 26(9) , 909-19, (1996)] |
|
Retinoids and related compounds. XI. Synthesis and stereoche...
1988-09-01 [Chem. Pharm. Bull. 36(9) , 3328-40, (1988)] |
|
The ORF slr0091 of Synechocystis sp. PCC6803 encodes a high-...
2013-08-01 [FEBS J. 280(15) , 3685-96, (2013)] |
|
Peroral challenge tests with food additives in urticaria and...
1986-04-01 [Int. J. Dermatol. 25(3) , 178-80, (1986)] |