Kinetic deuterium isotope effects on the N-demethylation of tertiary amides by cytochrome P-450.
L R Hall, R P Hanzlik
文献索引:J. Biol. Chem. 265(21) , 12349-55, (1990)
全文:HTML全文
摘要
Liver microsomal cytochrome P-450 readily N-dealkylates N,N-dimethylamides. N-Methyl-N-hydroxymethyl amides were isolated as intermediates and characterized by gas chromatography-mass spectrometry as their trimethylsilyl ethers. Intramolecular kinetic deuterium isotope effects measured for the enzymic N-demethylation of a series of 12 aromatic and aliphatic N-methyl-N-trideuteriomethyl amides, RCON(CH3)CD3, varied from 3.6 to 6.9 but were independent of both amide bond rotation rate and substrate oxidation potential. These values, which represent a lower limit to the intrinsic isotope effect (Dkintrinsic), are significantly larger than those observed for anodic N-demethylation and are consistent with a mechanism involving hydrogen atom abstraction. On the other hand, with N,N-dimethylbenzamide the intermolecular kinetic deuterium isotope effects on Vmax and Vmax/Km were found to be much smaller (1.23 and 1.75, respectively) indicating substantial suppression of the intrinsic isotope effect. Such suppression indicates the occurrence of a rate-limiting step other than the isotopically sensitive step together with a strong commitment to catalysis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
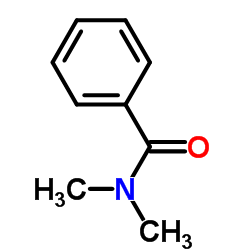 |
N,N-二甲基苯甲酰胺
CAS:611-74-5 |
C9H11NO |
|
The formation and metabolism of N-hydroxymethyl compounds--I...
1983-06-01 [Biochem. Pharmacol. 32(11) , 1773-81, (1983)] |
|
Hydrotropic polymer micelles as versatile vehicles for deliv...
2011-05-30 [J. Control. Release 152(1) , 13-20, (2011)] |
|
Hydrotropic solubilization of poorly water-soluble drugs.
2010-09-01 [J. Pharm. Sci. 99(9) , 3953-65, (2010)] |
|
Reactions of ethyl-and phenyllanthanide s complexes with n, ...
[Polyhedron 2(10) , 1101-02, (1983)] |
|
Deuterium exchange labelling of substituted aromatics using ...
[J. Labelled Comp. Radiopharm. 33(5) , 431-8, (1993)] |