Steric and electronic effects in the host-guest hydrogen bonding in clathrate hydrates.
Waldemar Kulig, Piotr Kubisiak, Lukasz Cwiklik
文献索引:J. Phys. Chem. A 115(23) , 6149-54, (2011)
全文:HTML全文
摘要
Clathrate hydrates with polar guest molecules (dimethyl ether, ethylene oxide, trimethylene oxide, tetrahydrofuran, and tetrahydropyran) were studied by means of the density functional theory. A model of a large cage of structure-I clathrate was employed. Optimal configurations of encaged guests were investigated with a focus on the host-guest hydrogen bond formation. Weak hydrogen bonds were found to be formed by each guest, while for THP a strong hydrogen bond and formation of L-defect was also observed. This is in accord with previous computational and experimental studies. Steric factors were shown to play a key role for the strength of the hydrogen bond formed. Interestingly, the host-guest binding is influenced not only by the size of a guest molecule but also by its shape. This work demonstrates that both electronic and steric properties of a polar guest should be considered for a full description of clathrate systems.© 2011 American Chemical Society
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
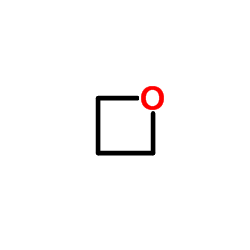 |
三甲氧基酯
CAS:503-30-0 |
C3H6O |
|
Synthesis of highly substituted oxetanes via [2+2] cycloaddi...
2013-04-11 [Chem. Commun. (Camb.) 49(28) , 2930-2, (2013)] |
|
A selective intramolecular transacylation of taxoids accompa...
2012-01-01 [Chem. Pharm. Bull. 60(3) , 415-8, (2012)] |
|
Three-component assembly and divergent ring-expansion cascad...
2010-11-22 [Angew. Chem. Int. Ed. Engl. 49(48) , 9210-4, (2010)] |
|
A theoretical rationale for why azetidine has a faster rate ...
2011-08-11 [J. Phys. Chem. B 115(31) , 9681-6, (2011)] |
|
Chemoassay screening of DNA-reactive mutagenicity with 4-(4-...
2012-10-15 [Chem. Res. Toxicol. 25(10) , 2092-102, (2012)] |