Effect of probenecid on the pharmacokinetics of carbamazepine in healthy subjects.
Kyoung-Ah Kim, Sae Ock Oh, Pil-Whan Park, Ji-Young Park
文献索引:Eur. J. Clin. Pharmacol. 61(4) , 275-80, (2005)
全文:HTML全文
摘要
Carbamazepine (CBZ) undergoes biotransformation by CYP3A4 and CYP2C8, and glucuronide conjugation. There has been no clear demonstration to reveal the role of glucuronidation in the disposition of CBZ. We evaluated the effect of probenecid, a UDP-glucuronosyltransferase inhibitor, on the pharmacokinetics of CBZ in humans.In a randomized, open-label, two-way crossover study, ten healthy male subjects were treated twice daily for 10 days with 500 mg probenecid or with a matched placebo. On day 6, a single dose of 200 mg CBZ was administered orally. Concentrations of CBZ and CBZ 10,11-epoxide (CBZ-E) in plasma and urine were measured.Probenecid decreased the area under the plasma concentration-time curve (AUC) of CBZ from 1253.9 micromol h/l to 1020.7 micromol h/l (P < 0.001) while increasing that of CBZ-E from 137.6 micromol h/l to 183.5 micromol h/l (P = 0.033). The oral clearance of CBZ was increased by probenecid by 26% (90% confidence interval, 17-34%; P < 0.001). Probenecid increased the AUC ratio of CBZ-E/CBZ from 0.11 to 0.16 (P < 0.001). However, probenecid had minimal effect on the recovery of the conjugated and free forms of CBZ and CBZ-E in urine.Although probenecid showed a minimal effect on the glucuronidation of CBZ and CBZ-E, it increased CBZ biotransformation to CBZ-E, most likely reflecting the induction of CYP3A4 and CYP2C8 activities, in humans. These results demonstrate that glucuronide conjugation plays a minor role in the metabolism of CBZ and CBZ-E in humans, and that probenecid has an inducing effect on the disposition of CBZ.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
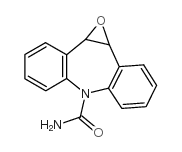 |
卡马西平 10,11-环氧化物
CAS:36507-30-9 |
C15H12N2O2 |
|
Influence of CYP3A4 induction/inhibition on the pharmacokine...
2014-11-01 [Clin. Ther. 36(11) , 1638-49, (2014)] |
|
Evaluation of the potential for pharmacokinetic drug-drug in...
2015-02-01 [Clin. Ther. 37(2) , 325-37, (2015)] |
|
Successful treatment of carbamazepine poisoning with hemodia...
2011-07-01 [Hemodial. Int. 15(3) , 407-11, (2011)] |
|
Effects of carbamazepine and metabolites on IL-2, IL-5, IL-6...
2011-01-01 [Pharmacol. Rep. 63(1) , 86-94, (2011)] |
|
Chemometric resolution of fully overlapped CE peaks: quantit...
2008-11-01 [Electrophoresis 29(22) , 4527-37, (2008)] |