A catalytic reactor for the organocatalyzed enantioselective continuous flow alkylation of aldehydes.
Riccardo Porta, Maurizio Benaglia, Alessandra Puglisi, Alessandro Mandoli, Andrea Gualandi, Pier Giorgio Cozzi
文献索引:ChemSusChem 7(12) , 3534-40, (2014)
全文:HTML全文
摘要
The use of immobilized metal-free catalysts offers the unique possibility to develop sustainable processes in flow mode. The challenging intermolecular organocatalyzed enantioselective alkylation of aldehydes was performed for the first time under continuous flow conditions. By using a packed-bed reactor filled with readily available supported enantiopure imidazolidinone, different aldehydes were treated with three distinct cationic electrophiles. In the organocatalyzed α-alkylation of aldehydes with 1,3-benzodithiolylium tetrafluoroborate, excellent enantioselectivities, in some cases even better than those obtained in the flask process (up to 95% ee at 25 °C), and high productivity (more than 3800 h(-1) ) were obtained, which thus shows that a catalytic reactor may continuously produce enantiomerically enriched compounds. Treatment of the alkylated products with Raney-nickel furnished enantiomerically enriched α-methyl derivatives, key intermediates for active pharmaceutical ingredients and natural products.© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
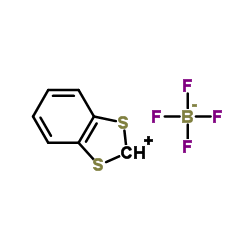 |
1,3-苯并二硫代吡咯四氟化硼盐
CAS:57842-27-0 |
C7H5BF4S2 |
|
Highly enantioselective α alkylation of aldehydes with 1,3-b...
2011-08-16 [Angew. Chem. Int. Ed. Engl. 50(34) , 7842-6, (2011)] |
|
Triclinic polymorph of dibenzotetra-thia-fulvalene.
2009-01-01 [Acta Crystallogr. Sect. E Struct. Rep. Online 65(Pt 9) , o2083, (2009)] |
|
The Facile and Direct Formylation of Organoboron Aromatic Co...
[European J. Org. Chem. 2013(22) , 4909-4917, (2013)] |
|
1, 3-Benzodithiolylium salts. Synthesis and reactions with n...
[Bull. Chem. Soc. Jpn. 49(12) , 3567-3573, (1976)] |
|
Adsorption of 1, 3-benzodithiolylium tetrafluoroborate (1, 3...
[J. Korean Phys. Soc. 57(1) , 1-4, (2010)] |