New potent 5-HT(2A) receptor ligands containing an N'-cyanopicolinamidine nucleus: Synthesis and in vitro pharmacological evaluation.
Ferdinando Fiorino, Beatrice Severino, Elisa Magli, Elisa Perissutti, Francesco Frecentese, Antonella Esposito, Giuseppina Maria Incisivo, Antonio Ciano, Paola Massarelli, Cristina Nencini, Vincenzo Santagada, Giuseppe Caliendo
文献索引:Eur. J. Med. Chem. 47(1) , 520-9, (2012)
全文:HTML全文
摘要
N'-cyanopicolinamidine derivatives, linked to an arylpiperazine moiety, were prepared and their affinity to serotonin 5-HT(1A), 5-HT(2A) and 5-HT(2C) receptors were evaluated. The combination of structural elements (heterocyclic nucleus, alkyl chain and 4-substituted piperazine) known to be critical for affinity to 5-HT(1A) receptors and the proper selection of substituents led to compounds with high specificity and affinity towards serotoninergic receptors. In binding studies, several molecules showed affinity in nanomolar and subnanomolar range at 5-HT(2A) and moderate to no affinity for other relevant receptors (5-HT(1A), 5-HT(2C), D(1), D(2), α(1) and α(2)). N'-cyano-N-(3-(4-(3-chlorophenyl)piperazin-1-yl)propyl)-picolinamidine (4l) with K(i)=0.000185nM, was the most active and selective derivative for the 5-HT(2A) receptor compared to other serotoninergic, dopaminergic and adrenergic receptors.Copyright © 2011 Elsevier Masson SAS. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
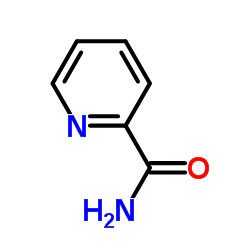 |
吡啶酰胺
CAS:1452-77-3 |
C6H6N2O |
|
Quantification of Sorafenib in Human Serum by Competitive En...
2015-01-01 [Biol. Pharm. Bull. 38 , 1788-93, (2015)] |
|
Regulation of synaptic MAPK/ERK phosphorylation in the rat s...
2015-10-01 [J. Neurosci. Res. 93 , 1592-9, (2015)] |
|
Self-assembled hybrid metal oxide base catalysts prepared by...
2015-01-01 [Nat. Commun. 6 , 8580, (2015)] |
|
Isolation of oxidative degradation products of atorvastatin ...
2015-12-01 [Biomed. Chromatogr. 29 , 1901-6, (2015)] |
|
First Novozym 435 lipase-catalyzed Morita-Baylis-Hillman rea...
2016-03-01 [Enzyme Microb. Technol. 84 , 32-40, (2016)] |