Interaction of nicotinamide and picolinamide with phosphatidylcholine and phosphatidylethanolamine membranes: a combined approach using dipole potential measurements and quantum chemical calculations.
Ana Borba, Fabiana Lairion, Anibal Disalvo, Rui Fausto
文献索引:Biochim. Biophys. Acta 1788(12) , 2553-62, (2009)
全文:HTML全文
摘要
Interaction between the bioactive compounds nicotinamide and picolinamide and phospholipids (phosphatidylcholines and phosphatidylethanolamines) was investigated by a combined approach using dipole potential measurements and quantum chemical calculations. It is shown that nicotinamide and picolinamide interactions with phosphatidylcholines are of two main types: (i) specific interactions with the phosphate group of the lipid, for which H-bonding between NH(2) group of the substrate and the phosphate plays a dominant role, (ii) conjugated less specific weaker interactions involving both the phosphate and carbonyl groups of the head group, which propagate to the lipid alkyl chains and increase their conformational disorder. For phosphatidylethanolamines, picolinamide was found to decrease the dipole potential of the membrane in a similar way as for phosphatidylcholines, while nicotinamide is ineffective. These findings are correlated with the specific properties of phosphatidylethanolamines (reduced exposure of phosphate groups) and structural differences in the two substrates, in particular: different separation of the nitrogen atoms in the molecules, existence of a strong intramolecular hydrogen bond in picolinamide (NH...N ((ring))), which is absent in nicotinamide, and non-planarity of nicotinamide molecules, in contrast to picolinamide ones. Additional information on the lipid/substrate interactions was extracted from the analysis of the changes produced in the relevant vibrational frequencies of the lipid and substrate upon binding. The present study gives molecular support to the argument that changes of dipole potentials are due to effects on the constitutive dipolar PO and CO groups. In addition, it is also shown that according to the specific binding of the substrate to one or both of those, the conformational state of the acyl chains may be affected. These entropy effects may be in the origin of the well-known interdependence of the properties of one monolayer with respect to the other in bilayer membranes.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
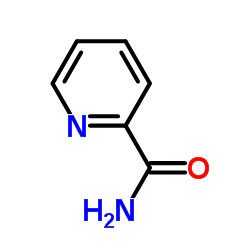 |
吡啶酰胺
CAS:1452-77-3 |
C6H6N2O |
|
Quantification of Sorafenib in Human Serum by Competitive En...
2015-01-01 [Biol. Pharm. Bull. 38 , 1788-93, (2015)] |
|
Regulation of synaptic MAPK/ERK phosphorylation in the rat s...
2015-10-01 [J. Neurosci. Res. 93 , 1592-9, (2015)] |
|
Self-assembled hybrid metal oxide base catalysts prepared by...
2015-01-01 [Nat. Commun. 6 , 8580, (2015)] |
|
Isolation of oxidative degradation products of atorvastatin ...
2015-12-01 [Biomed. Chromatogr. 29 , 1901-6, (2015)] |
|
First Novozym 435 lipase-catalyzed Morita-Baylis-Hillman rea...
2016-03-01 [Enzyme Microb. Technol. 84 , 32-40, (2016)] |