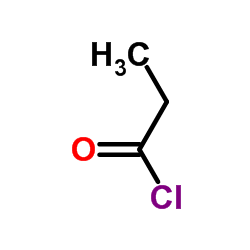
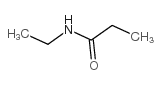
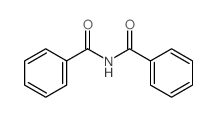
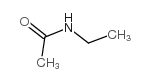
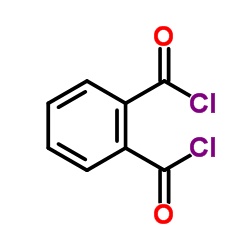
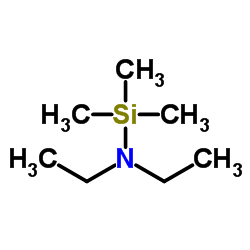
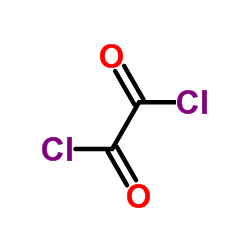
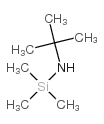
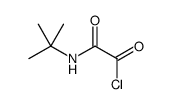
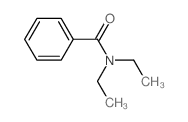
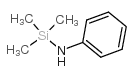
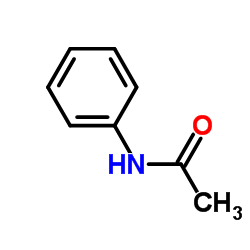
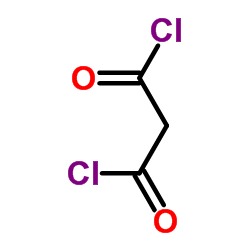
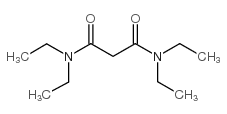
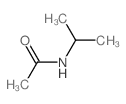
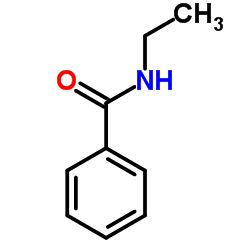
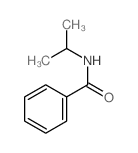
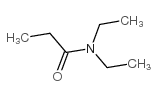
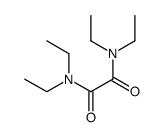
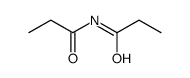
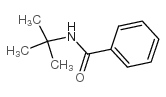
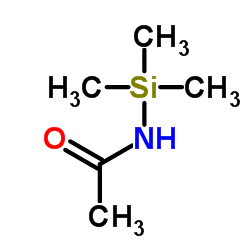
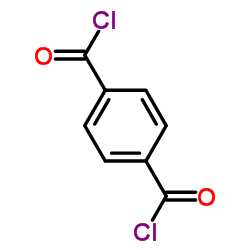
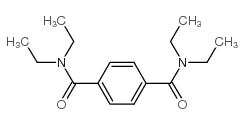
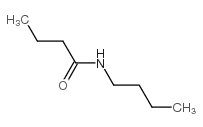
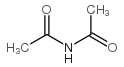
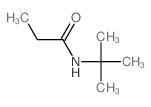
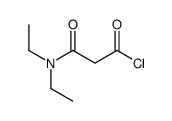
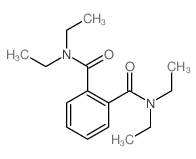
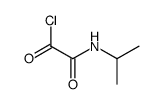
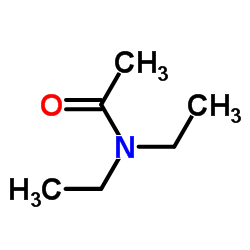
|
~69% |
|
~52% |
|
~68% |
|
~31% |
|
~41% |
|
~69% |
|
~62% |
|
~67% |
|
~81% |
|
~76% |
|
~86% |
|
~68% |
|
~59% |
|
~47% |
|
~65% |
|
~86% |
|
~72% |
|
~36% |
|
~86% |
|
~52% |
|
~71% |
|
~76% |
|
~39% |
|
~40% |
|
~69% |
|
~34% |
|
~67% |