| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
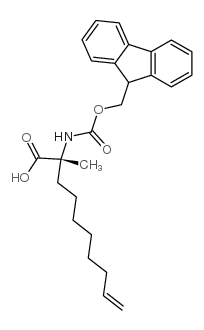 |
(2R)-2-N-芴甲氧羰基氨基-2-甲基-9-癸烯酸
CAS:945212-26-0 |
|
 |
N-芴甲氧羰基-alpha-烯丙基-L-丙氨酸
CAS:288617-71-0 |