Biochemical and Biophysical Research Communications
1987-08-31
The purification and subunit structure of a membrane-bound ATPase from the Archaebacterium Halobacterium saccharovorum.
L I Hochstein, H Kristjansson, W Altekar
文献索引:Biochem. Biophys. Res. Commun. 147 , 295-300, (1987)
全文:HTML全文
摘要
A membrane-bound ATPase from Halobacterium saccharovorum was solubilized using sodium deoxycholate and Zwittergent 3-10 and purified by hydrophobic and ammonium sulfate-mediated chromatography. The enzyme, which had a molecular mass of 350 kDa, was composed of two major (87 and 60 kDa) and two minor (29 kDa and 20 kDa) subunits. The halobacterial ATPases appear to be unlike any other ATPase described to date.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
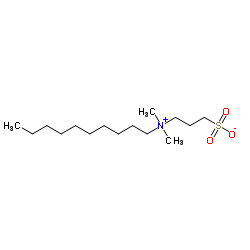 |
3-(癸基二甲基铵)丙烷-1-磺酸内盐
CAS:15163-36-7 |
C15H33NO3S |
相关文献:
更多...
|
Multi-target spectral moment QSAR versus ANN for antiparasit...
2010-03-15 [Bioorg. Med. Chem. 18 , 2225-31, (2010)] |
|
Characterizing synthetic polymers and additives using new io...
2014-06-15 [Rapid Commun. Mass Spectrom. 28(11) , 1175-84, (2014)] |
|
The hop-like stress-induced protein 1 cochaperone is a novel...
2014-08-01 [J. Virol. 88(16) , 9361-78, (2014)] |
|
Isolation and preparation of chloroplasts from Arabidopsis t...
2008-01-01 [Methods Mol. Biol. 425 , 171, (2008)] |
|
A comparative analysis of polyurethane hydrogel for immobili...
2007-06-05 [Anal. Chim. Acta 592 , 132, (2007)] |