Efficacy, safety and risk of augmentation of rotigotine for treating restless legs syndrome.
Yuichi Inoue, Koichi Hirata, Kenichi Hayashida, Nobutaka Hattori, Takayuki Tomida, Diego Garcia-Borreguero
文献索引:Prog. Neuropsychopharmacol. Biol. Psychiatry 40 , 326-33, (2013)
全文:HTML全文
摘要
The present study aimed to examine the long-term efficacy and safety of rotigotine treatment for restless legs syndrome (RLS), as well as the rate of clinically significant augmentation, in a 1-year open-label study of Japanese subjects. Japanese patients with RLS who had been treated with rotigotine or placebo in a double-blind trial were enrolled in a 1-year, open-label, uncontrolled extension study and treated with rotigotine at a dose of up to 3 mg/24 h after an 8-week titration phase. Outcomes included International Restless Legs Syndrome Study Group rating scale (IRLS scale), Pittsburgh Sleep Quality Index (PSQI), safety, and investigator-/expert panel-assessed augmentation (including Augmentation Severity Rating Scale). Overall, 185 patients entered the open-label study and 133 completed the study. IRLS and PSQI total scores improved throughout the 52-week treatment period (IRLS, from 23.2±5.1 to 7.8±7.6 and PSQI, from 8.0±3.1 to 5.0±2.9). Treatment-emergent adverse events were mild to moderate in severity, and included application site reactions (52.4%) and nausea (28.6%). Clinically significant augmentation occurred in five patients (2.7%). These results indicate a good long-term efficacy of rotigotine for treating RLS, with a relatively low risk of clinically significant augmentation.Copyright © 2012 Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
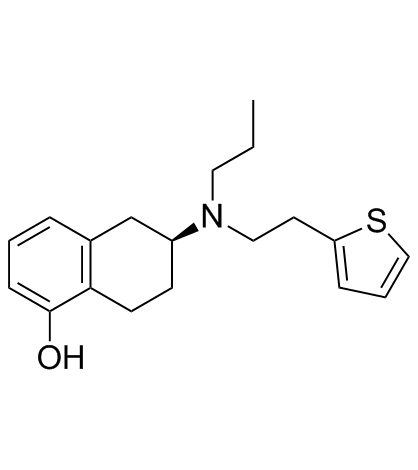 |
罗替高汀
CAS:99755-59-6 |
C19H25NOS |
|
Three-month subchronic intramuscular toxicity study of rotig...
2013-02-01 [Food Chem. Toxicol. 52 , 143-52, (2013)] |
|
Medication update.
2012-06-10 [Nurse Pract. 37(6) , 56, (2012)] |
|
ADMET considerations for restless leg syndrome drug treatmen...
2012-10-01 [Expert Opin. Drug Metab. Toxicol. 8(10) , 1247-61, (2012)] |
|
Impulse control disorder in patients with Parkinson's diseas...
2014-08-01 [J. Neurol. Neurosurg. Psychiatr. 85(8) , 840-4, (2014)] |
|
Breakthrough symptoms during the daytime in patients with re...
2012-02-01 [Sleep Med. 13(2) , 151-5, (2012)] |