Assessment of cell-signaling pathways in the regulation of mammalian target of rapamycin (mTOR) by amino acids in rat adipocytes.
P T Pham, S J Heydrick, H L Fox, S R Kimball, L S Jefferson, C J Lynch
文献索引:J. Cell. Biochem. 79(3) , 427-41, (2000)
全文:HTML全文
摘要
Enhanced phosphorylation of the ribosomal protein s6 kinase, p70(s6k), and the translational repressor, 4E-BP1, are associated with either insulin-induced or amino acid-induced protein synthesis. Hyperphosphorylation of p70(s6k) and 4E-BP1 in response to insulin or amino acids is mediated through the mammalian target of rapamycin (mTOR). In several cell lines, mTOR or its downstream targets can be regulated by phosphatidylinositol (PI) 3-kinase; protein kinases A, B, and C; heterotrimeric G-proteins; a PD98059-sensitive kinase or calcium; as well as by amino acids. Regulation by amino acids appears to involve detection of levels of charged t-RNA or t-RNA synthetase activity and is sensitive to inhibition by amino acid alcohols. In the present article, however, we show that the rapamycin-sensitive regulation of 4E-BP1 and p70(s6k) in freshly isolated rat adipocytes is not inhibited by either L-leucinol or L-histidinol. This finding is in agreement with other recent studies from our laboratory suggesting that the mechanism by which amino acids regulate mTOR in freshly isolated adipocytes may be different than the mechanism found in a number of cell lines. Therefore we investigated the possible role of growth factor-regulated and G-protein-regulated signaling pathways in the rapamycin-sensitive, amino acid alcohol-insensitive actions of amino acids on 4E-BP1 phosphorylation. We found, in contrast to previously published results using 3T3-L1 adipocytes or other cell lines, that the increase in 4E-BP1 phosphorylation promoted by amino acids was insensitive to agents that regulate protein kinase A, mobilize calcium, or inhibit protein kinase C. Furthermore, amino acid-induced 4E-BP1 phosphorylation was not blocked by pertussis toxin nor was it mimicked by the G-protein agonists fluoroaluminate or MAS-7. However, amino acids failed to activate either PI 3-kinase, protein kinase B, or mitogen-activated protein kinase and failed to promote tyrosine phosphorylation of cellular proteins, similar to observations made using cell lines. In summary, amino acids appear to use an amino acid alcohol-insensitive mechanism to regulate mTOR in freshly isolated adipocytes. This mechanism is independent of cell-signaling pathways implicated in the regulation of mTOR or its downstream targets in other cells. Overall, our study emphasizes the need for caution when extending results obtained using established cell lines to the differentiated nondividing cells found in most tissues.Copyright 2000 Wiley-Liss, Inc.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
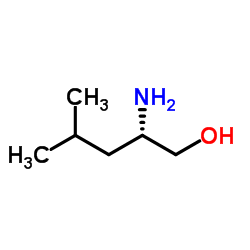 |
L-亮氨醇
CAS:7533-40-6 |
C6H15NO |
|
Pharmaceuticals and Surfactants from Alga-Derived Feedstock:...
2015-08-24 [ChemSusChem 8 , 2670-80, (2015)] |
|
Chiral bis(amino alcohol)oxalamide gelators-gelation propert...
2003-11-21 [Chemistry 9(22) , 5567-80, (2003)] |
|
A colorimetric chiral sensor based on chiral crown ether for...
2011-04-01 [Chirality 23(4) , 349-53, (2011)] |
|
Potent and systemically active aminopeptidase N inhibitors d...
1992-04-03 [J. Med. Chem. 35 , 1259, (1992)] |
|
Design and synthesis of some substrate analogue inhibitors o...
1992-08-07 [J. Med. Chem. 35 , 2939, (1992)] |