Laser-induced fluorescence spectra of 4-methylcyclohexoxy radical and perdeuterated cyclohexoxy radical and direct kinetic studies of their reactions with O2.
Lei Zhang, Karen M Callahan, Dean Derbyshire, Theodore S Dibble
文献索引:J. Phys. Chem. A 109(41) , 9232-40, (2005)
全文:HTML全文
摘要
The laser-induced fluorescence (LIF) excitation spectra of the 4-methylcyclohexoxy and d11-cyclohexoxy radicals have been measured for the first time. LIF intensity was used as a probe in direct kinetic studies of the reaction of O(2) with trans-4-methylcyclohexoxy and d11-cyclohexoxy radicals from 228 to 301 K. Measured rate constants near room temperature are uniformly higher than the Arrhenius fit to the lower-temperature data, which can be explained by the regeneration of cyclic alkoxy radicals from the product of their beta-scission and the effect of O(2) concentration on the extent of regeneration. The Arrhenius expressions obtained over more limited ranges were k(O2) = (1.4(+8)(-1)) x 10(-13) exp[(-810 +/- 400)/T] cm(3) molecule(-1) s(-1) for trans-4-methylcyclohexoxy (228-292 K) and k(O2) = (3.7(+4)(-1)) x 10(-14) exp )[(-760 +/- 400) /T] cm(3) molecule(-1) s(-1) for d11-cyclohexoxy (228-267 K) independent of pressure in the range 50-90 Torr. The room-temperature rate constant for the reaction of trans-4-methylcyclohexoxy radical with O2 (obtained from the Arrhenius fit) is consistent with the commonly recommended value, but the observed activation energy is approximately 3 times larger than the recommended value of 0.4 kcal/mol and half the value previously found for the reaction of normal cyclohexoxy radical with O2.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
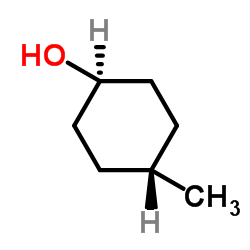 |
trans-4-甲基环己醇
CAS:7731-29-5 |
C7H14O |
|
Behavioral responses to odorants in drosophila require nervo...
2006-09-01 [Chem. Senses 31(7) , 627-39, (2006)] |
|
Studies of 4-methylcyclohexanol: an Aedes triseriatus (Dipte...
1982-10-14 [J. Med. Entomol. 19(5) , 589-92, (1982)] |
|
Laboratory tests of the effects of p-cresol and 4-methylcycl...
1989-10-01 [Med. Vet. Entomol. 3(4) , 347-52, (1989)] |
|
Drosophila olfactory response rhythms require clock genes bu...
2005-06-01 [J. Biol. Rhythms 20(3) , 237-44, (2005)] |
|
Attention deficit induced by blockade of N-methyl D-aspartat...
2011-03-10 [Gene 245(1) , 31-42, (2000)] |