Physicochemical characterization and transformation of the cytosolic glucocorticoid receptor from rabbit liver.
P Blanchardie, P Lustenberger, J L Orsonneau, S Bernard
文献索引:J. Steroid Biochem. 18(6) , 789-99, (1983)
全文:HTML全文
摘要
Characterization of the glucocorticoid receptor from rabbit liver cytosol was studied in vitro. Binding of [3H]-dexamethasone showed a high affinity (KD = 2.4 . 10(-9)M) and a concentration of binding sites of 0.3 . 10(-12) mol/mg proteins. Association and dissociation rate constants for [3H]-dexamethasone were respectively 1.6 . 10(5)M-1 . min-1 and 2.5 . 10(-4) min-1. Competition experiments showed that dexamethasone and triamcinolone acetonide were the most effective competitors while progesterone and aldosterone competed very poorly. The stabilities of bound and unbound forms were investigated with and without molybdate and other oxyanions. Calibrated gel filtration gave a stokes radius of 5.6 nm and an apparent mol. wt of 280,000. The untransformed complex sedimented at 9 s in 5-20% sucrose density gradients and the transformed species sedimented near 4 s. The behaviour of the three forms of receptor, untransformed, transformed and molybdate-stabilized was studied on ionic exchangers. Transformation of the [3H]-dexamethasone--receptor complex was shown to occur both with high ionic strength and elevated temperature. The transformation step was almost completely inhibited by tungstate and molybdate while vanadate revealed a very slight inhibitory effect.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
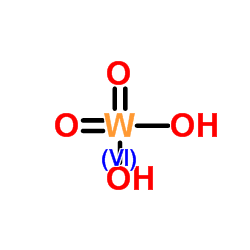 |
钨酸
CAS:7783-03-1 |
H2O4W |
|
Catalytic conversion of cellulose to ethylene glycol over a ...
2013-04-01 [ChemSusChem 6(4) , 652-8, (2013)] |
|
Identification in various chlorate-resistant mutants of a pr...
1985-04-17 [Biochim. Biophys. Acta 839(2) , 181-90, (1985)] |
|
Effect of oxyanions of the early transition metals on rabbit...
1983-10-11 [Biochemistry 22(21) , 4994-5000, (1983)] |
|
Interaction between isopoly-tungstic acid and berberine hydr...
2003-07-01 [Anal. Sci. 19(7) , 1055-60, (2003)] |
|
Vanadate, tungstate and molybdate activate rod outer segment...
1985-04-22 [Biochim. Biophys. Acta 845(1) , 81-5, (1985)] |