Characterization of Leydig cell gonadotropin-releasing hormone binding sites utilizing radiolabeled agonist and antagonist.
R P Millar, A Garritsen, E Hazum
文献索引:Peptides 3(5) , 789-92, (1982)
全文:HTML全文
摘要
Gonadotropin-releasing hormone (GnRH) binding sites in intact Leydig cells and in membrane preparations were investigated using 125I-labeled GnRH agonist and antagonist. Binding was saturable and involved a single class of high affinity sites. Intact Leydig cells and a membrane preparation had a higher affinity for GnRH agonist (Kd 3.0 +/- 1.7 X 10(-10) M) than for GnRH antagonist (Kd 10.0 +/- 1.8 X 10(-10) M). With anterior pituitary membranes the Kd was 2.8 +/- 0.7 X 10(-10) M for the agonist and 2.4 +/- 1.4 X 10(-10) M for the antagonist. The Kd for GnRH was similar for Leydig cells and the anterior pituitary. Chymotrypsin and trypsin digestion decreased receptor binding, but neuraminidase increased Leydig cell binding in contrast to the decrease in binding observed with pituitary receptors. The results suggest that the Leydig cell GnRH binding sites may differ from the pituitary receptor which may be related to structural differences in GnRH-like peptides recently described in extracts of rat testis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
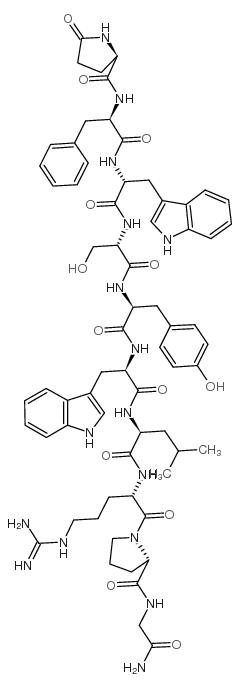 |
(D-PYR1,D-PHE2,D-TRP3·6)-LHRH
CAS:68059-94-9 |
C67H84N16O13 |
|
Role of lipids in gonadotropin releasing hormone agonists an...
1982-03-15 [Biochem. Biophys. Res. Commun. 105(1) , 8-13, (1982)] |
|
LH-RH analogue acts as substance P antagonist by inhibiting ...
1985-07-01 [Brain Res. 337(2) , 357-61, (1985)] |
|
Indirect evidence for a physiological role exerted by a "tes...
1990-07-01 [Gen. Comp. Endocrinol. 79(1) , 147-53, (1990)] |
|
Jun localization in cytosolic and nuclear compartments in br...
2004-02-01 [Gen. Comp. Endocrinol. 135(3) , 310-23, (2004)] |
|
Microinjection of LHRH and its antagonistic analog into the ...
1994-10-01 [Endocr. J. 41(5) , 559-63, (1994)] |