Chiral capillary electrophoretic analysis of verapamil metabolism by cytochrome P450 3A4.
Pham Thi Thanh Ha, Inge Sluyts, Sigrid Van Dyck, Jie Zhang, Ron A H J Gilissen, Jos Hoogmartens, Ann VanSchepdael
文献索引:J. Chromatogr. A. 1120(1-2) , 94-101, (2006)
全文:HTML全文
摘要
Cytochrome P450 (CYP), which is one of the most important enzymes in human liver, is responsible for a large portion of the first-pass metabolism of drugs. Many studies have focused on the determination of CYP activity by substrate assays. Most of them used liquid chromatography (LC) as analytical technique, while only a few studies used capillary electrophoresis (CE) for the separation and quantitation of reaction components. In this study, the feasibility of using CE in an in vitro metabolism study with CYP was tested. Verapamil was chosen as the substrate for CYP 3A4 isozyme (Supersome). A chiral capillary electrophoretic method was developed and validated for the simultaneous determination of R,S-verapamil (VER) and their major metabolites, R,S-norverapamil (NOR). A method for CYP 3A4 activity assay was proposed with VER as a probe. At the same time, the enantioselective metabolism of VER was studied. Michaelis-Menten constants of R- and S-VER were determined. S-VER was metabolised faster and more extensively than R-VER, with K(m)=167+/-23 microM, V(max)=3,418+/-234 pmol/min/mg for S-VER, and K(m)=168+/-35 microM, V(max)=2,502+/-275 pmol/min/mg for R-VER.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
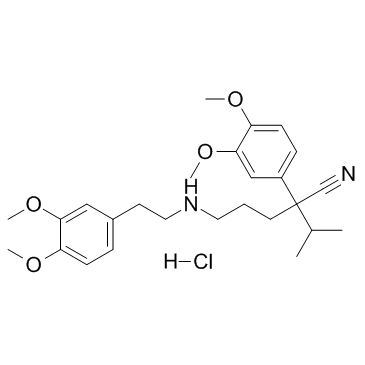 |
盐酸去甲维拉帕米
CAS:67812-42-4 |
C26H37ClN2O4 |
|
Verapamil, and its metabolite norverapamil, inhibit macropha...
2014-08-01 [J. Infect. Dis. 210(3) , 456-66, (2014)] |
|
Application of permeability-limited physiologically-based ph...
2013-09-01 [J. Pharm. Sci. 102(9) , 3161-73, (2013)] |
|
Stereoselective CZE method for analysis of verapamil and nor...
2013-01-01 [Acta Pol. Pharm. 70(3) , 395-401, (2013)] |
|
Comparative cardiovascular actions of verapamil and its majo...
1978-04-01 [Cardiovasc. Res. 12 , 247-254, (1978)] |
|
The pharmacology of verapamil. V. Tissue distribution of ver...
1983-01-01 [Pharmacology 27 , 1-8, (1983)] |