Aspirin--a national survey III: Determination of impurities in bulk aspirin and aspirin formulations by high-pressure liquid chromatography and spectrophotometry.
R D Kirchhoefer, J C Reepmeyer, W E Juhl
文献索引:J. Pharm. Sci. 69(5) , 550-3, (1980)
全文:HTML全文
摘要
A quantitative high-pressure liquid chromatographic method, using a reversed-phase column and an aqueous acetic acid-methanol solution as the mobile phase, was employed for the determination of O-acetyl-O-salicylsalicylic acid and O-salicylsalicylic acid in pharmaceutical aspirin preparations. The aspirin was dissolved, filtered, and injected into the chromatograph. The absorbance of the impurities was measured at 254 nm. Acetylsalicylic anhydridge was determined by a spectrophotometric method. The aspirin was dissolved in pH 11.3 buffer and extracted with benzene. An aliquot of the benzene was evaporated, and the residue was dissolved in alpha-benzamidocinnamate-pyridine reagent. The acetylsalicylic anhydride was measured using the difference between the absorbance at 362 and 372 nm. Possible interference of aspirin with the procedure is discussed. Thirty-four bulk aspirin and 172 tablet formulations were examined. Results for O-acetyl-O-salicylsalicylic acid, O-salicylsalicylic acid and acetylsalicylic anhydride are given.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
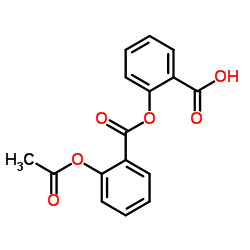 |
乙酰水杨酰水杨酸
CAS:530-75-6 |
C16H12O6 |
|
[A new possible strategy for prevention and preventive treat...
2009-03-15 [Orv. Hetil. 150(11) , 503-12, (2009)] |
|
Solid-state stability of aspirin in the presence of excipien...
1982-10-01 [J. Pharm. Sci. 71(10) , 1096-101, (1982)] |
|
The quantitative determination of aspirin and its degradatio...
1995-02-01 [J. Pharm. Biomed. Anal. 13(2) , 111-9, (1995)] |
|
Efficacy of 1,000 mg effervescent acetylsalicylic acid and s...
2004-01-01 [Eur. Neurol. 52(1) , 50-6, (2004)] |
|
Measurement of primary haemostasis using a pressure clamp te...
2001-12-01 [Platelets 12(8) , 462-70, (2001)] |