Evaluation of in vitro dissolution and in vivo oral absorption of dutasteride-loaded eudragit E nanoparticles.
M-S Kim
文献索引:Drug Res. (Stuttg.) 63(6) , 326-30, (2013)
全文:HTML全文
摘要
The present study sought to evaluate the pharmacokinetics of dutasteride-loaded Eudragit E nanoparticle in rats. In addition, the study investigated the effect of increasing drug load on the in vitro solubility and dissolution behavior of dutasteride together with its in vivo oral absorption characteristics. The suspension of dutasteride-loaded Eudragit E nanoparticles prepared by the nanoprecipitation method showed blue opalescence and the particles were uniform in appearance. The entrapment efficiency and the mean particle size of these nanoparticles were in the range of 98.1-99.3% and 120.5-128.4 nm, respectively, and no significant difference in these parameters was observed between the nanoparticles in the sample. Eudragit E nanoparticles containing a drug load of 5% showed an increase in bioavailability by 550% as compared to dutasteride suspension. This finding is attributable to enhanced solubility and dissolution of dutasteride when formulated as nanoparticles. Furthermore, the oral absorption of dutasteride in rats increased as a function of the extent of supersaturation of dutasteride in Eudragit E nanoparticles. Therefore, the preliminary results from our study suggest that dutasteride-loaded Eudragit E nanoparticles may have significant potential for clinical application. © Georg Thieme Verlag KG Stuttgart · New York.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
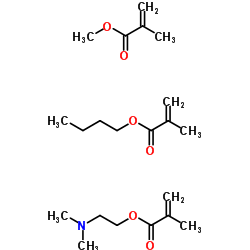 |
聚丙烯酸树脂Ⅳ
CAS:24938-16-7 |
C21H37NO6 |
|
Improved supersaturation and oral absorption of dutasteride ...
2012-01-01 [Chem. Pharm. Bull. 60(11) , 1468-73, (2012)] |
|
Effect of aminoalkyl methacrylate copolymer E/HCl on in vivo...
2013-11-01 [Drug Dev. Ind. Pharm. 39(11) , 1698-705, (2013)] |
|
Eudragit E as excipient for production of granules and table...
2007-01-01 [AAPS PharmSciTech 8(2) , Article 34, (2007)] |
|
Studies on gynaecological hydrophilic lactic acid preparatio...
2003-04-01 [Pharmazie 58(4) , 260-2, (2003)] |
|
In vitro evaluation of dissolution behavior for a colon-spec...
2002-01-01 [AAPS PharmSciTech 3(4) , E33, (2002)] |