[Chemical attachment of p-aminophenylgalactoside to acid alpha-glucosidase from human liver].
D M Belen'kiĭ, V I Mikhaĭlov, V N Shaptseva
文献索引:Biokhimiia 47(7) , 1141-6, (1982)
全文:HTML全文
摘要
Chemical modification of the COOH-groups of acid alpha-glucosidase from human liver by 1-ethyl-3 (3'-dimethylaminopropyl) carbodiimide. HCl in the presence of rho-aminophenyl-beta-D-galactopyranoside was carried out. The presence of covalently bound galactose derivative in the enzyme was followed by changes in the absorption spectra and electrophoretic mobility during polyacrylamide gel electrophoresis and by the ability of modified alpha-glucosidase to interact specifically with castor-bean lectin (RCA II). The modified enzyme retained its catalytic activity towards maltose and did not differ from native alpha-glucosidase in terms of its affinity for this substrate, the Km values for maltose during 5 and 6 mM, respectively. The in vitro changes in the marker specificity of acid alpha-glucosidase against the unchanged catalytic properties of the enzyme are discussed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
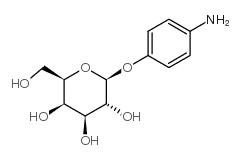 |
4-氨苯基β-D-吡喃半乳糖苷
CAS:5094-33-7 |
C12H17NO6 |
|
Amperometric detection of Enterobacteriaceae in river water ...
2010-09-16 [Anal. Chim. Acta 677 , 156-161, (2010)] |
|
Sugar coated liposomal flavonoid: a unique formulation in co...
2005-06-01 [J. Drug Target. 13(5) , 305-15, (2005)] |
|
Immunogenicity of galactosylated liposomes.
1984-01-01 [Immunol. Commun. 13(1) , 5-13, (1984)] |
|
Effect of membrane composition on the immune reactivity of g...
1984-06-01 [Indian J. Biochem. Biophys. 21(3) , 155-7, (1984)] |
|
Quantum dot based fluorometric detection of cancer TF-antige...
2013-10-15 [Anal. Chem. 85(20) , 9699-704, (2013)] |