Specific and reversible immobilization of proteins tagged to the affinity polypeptide C-LytA on functionalized graphite electrodes.
Daniel Bello-Gil, Beatriz Maestro, Jennifer Fonseca, Juan M Feliu, Víctor Climent, Jesús M Sanz
文献索引:PLoS ONE 9(1) , e87995, (2014)
全文:HTML全文
摘要
We have developed a general method for the specific and reversible immobilization of proteins fused to the choline-binding module C-LytA on functionalized graphite electrodes. Graphite electrode surfaces were modified by diazonium chemistry to introduce carboxylic groups that were subsequently used to anchor mixed self-assembled monolayers consisting of N,N-diethylethylenediamine groups, acting as choline analogs, and ethanolamine groups as spacers. The ability of the prepared electrodes to specifically bind C-LytA-tagged recombinant proteins was tested with a C-LytA-β-galactosidase fusion protein. The binding, activity and stability of the immobilized protein was evaluated by electrochemically monitoring the formation of an electroactive product in the enzymatic hydrolysis of the synthetic substrate 4-aminophenyl β-D-galactopyranoside. The hybrid protein was immobilized in an specific and reversible way, while retaining the catalytic activity. Moreover, these functionalized electrodes were shown to be highly stable and reusable. The method developed here can be envisaged as a general, immobilization procedure on the protein biosensor field.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
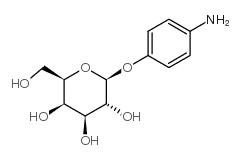 |
4-氨苯基β-D-吡喃半乳糖苷
CAS:5094-33-7 |
C12H17NO6 |
|
Amperometric detection of Enterobacteriaceae in river water ...
2010-09-16 [Anal. Chim. Acta 677 , 156-161, (2010)] |
|
Sugar coated liposomal flavonoid: a unique formulation in co...
2005-06-01 [J. Drug Target. 13(5) , 305-15, (2005)] |
|
Immunogenicity of galactosylated liposomes.
1984-01-01 [Immunol. Commun. 13(1) , 5-13, (1984)] |
|
Effect of membrane composition on the immune reactivity of g...
1984-06-01 [Indian J. Biochem. Biophys. 21(3) , 155-7, (1984)] |
|
Quantum dot based fluorometric detection of cancer TF-antige...
2013-10-15 [Anal. Chem. 85(20) , 9699-704, (2013)] |