Iridium-catalyzed addition of aroyl chlorides and aliphatic acid chlorides to terminal alkynes.
Tomohiro Iwai, Tetsuaki Fujihara, Jun Terao, Yasushi Tsuji
文献索引:J. Am. Chem. Soc. 2nd ed., 134 , 1268-1274, (2012)
全文:HTML全文
摘要
Iridium complexes show high catalytic activity in intermolecular additions of acid chlorides to terminal alkynes to afford valuable (Z)-β-chloro-α,β-unsaturated ketones. Ligands in the catalytic system play a crucial role in this reaction. An N-heterocyclic carbene (NHC) is an efficient ligand for the addition of aroyl chlorides, while dicyclohexyl(2-methylphenyl)phosphine (PCy(2)(o-Tol)) is indispensable for the reaction of aliphatic acid chlorides. The addition reactions proceed regio- and stereoselectively with suppression of decarbonylation and β-hydrogen elimination, which have been two major intrinsic problems in transition-metal-catalyzed reactions. Stoichiometric reactions of active iridium catalysts with aroyl chlorides and aliphatic acid chlorides are carried out to gain insights into the reaction mechanisms.© 2011 American Chemical Society
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
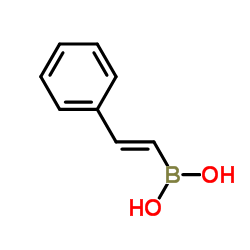 |
反式-BETA-苯乙烯硼酸
CAS:6783-05-7 |
C8H9BO2 |
|
Rh2(II)-catalyzed intramolecular aliphatic C-H bond aminatio...
2012-05-02 [J. Am. Chem. Soc. 17th ed., 134 , 7262-7265, (2012)] |
|
Copper-mediated sequential cyanation of aryl C-B and arene C...
2012-02-08 [J. Am. Chem. Soc. 5th ed., 134 , 2528-2531, (2012)] |
|
Ambient temperature synthesis of high enantiopurity N-protec...
2007-02-07 [J. Am. Chem. Soc. 129 , 1132, (2007)] |
|
Build/couple/pair strategy combining the Petasis 3-component...
2012-04-09 [ACS Comb. Sci. 4th ed., 14 , 253-257, (2012)] |
|
Rh(I)-catalyzed asymmetric 1,2-addition to α-diketones with ...
2012-01-20 [Org. Lett. 2nd ed., 14 , 624-627, (2012)] |