Analytical Biochemistry
1986-02-15
Single-step, quantitative derivatization of amino, carboxyl, and hydroxyl groups in iodothyronine amino acids with ethanolic pivalic anhydride containing 4-dimethylaminopyridine.
M Joppich, R Joppich-Kuhn, A Sentissi, R W Giese
文献索引:Anal. Biochem. 153(1) , 159-65, (1986)
全文:HTML全文
摘要
Reaction of thyroxine with ethanol and pivalic anhydride in the presence of 4-dimethylaminopyridine quantitatively forms N,O-dipivalyl thyroxine ethyl ester. Other iodothyronines react similarly and the procedure is moisture insensitive. Apparently this reaction is successful, in contrast to similar procedures reported for the derivatization of alpha-amino acids, because it overcomes the problem in other procedures of irreversible side reactions arising from an oxazolone intermediate.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
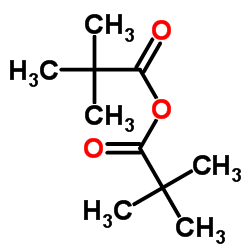 |
特戊酸酐
CAS:1538-75-6 |
C10H18O3 |
相关文献:
更多...
|
Kinetic resolution of racemic 2-hydroxy-γ-butyrolactones by ...
2013-03-15 [Org. Lett. 15(6) , 1170-3, (2013)] |
|
The concept of superactive esters. Could peptide synthesis b...
1994-03-01 [Int. J. Pept. Protein Res. 43 , 312, (1994)] |
|
Observation and elimination of N-acetylation of oligonucleot...
2001-05-07 [Bioorg. Med. Chem. Lett. 11(9) , 1105-1107, (2001)] |
|
New synthetic substrates of mammalian nucleotide excision re...
2013-07-01 [Nucleic Acids Res. 41 , e123, (2013)] |
|
Structural Basis for Substrate Specificity in Adenosylcobala...
2015-11-06 [J. Biol. Chem. 290 , 26882-98, (2015)] |