Journal of Organic Chemistry
2001-10-19
Rhodium-catalyzed asymmetric 1,4-addition of organoboron reagents to 5,6-dihydro-2(1H)-pyridinones. Asymmetric synthesis of 4-aryl-2-piperidinones.
T Senda, M Ogasawara, T Hayashi
文献索引:J. Org. Chem. 66(21) , 6852-6, (2001)
全文:HTML全文
摘要
Catalytic asymmetric synthesis of 4-aryl-2-piperidinones was realized for the first time by asymmetric 1,4-addition of arylboron reagents to 5,6-dihydro-2(1H)-pyridinones in the presence of a chiral bisphosphine-rhodium catalyst. In the reaction introducing 4-fluorophenyl group, the use of 4-fluorophenylboroxine and 1 equiv (to boron) of water at 40 degrees C gave the highest yield of the arylation product with high enantioselectivity (98% ee). The (R)-4-(4-fluorophenyl)-2-piperidinone obtained here is a key intermediate for the synthesis of (-)-Paroxetine.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
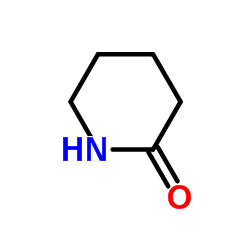 |
2-氮己环酮
CAS:675-20-7 |
C5H9NO |
相关文献:
更多...
|
The chemistry of escapin: identification and quantification ...
2009-01-01 [Chemistry 15(7) , 1597-603, (2009)] |
|
Biosynthesis of 5-aminopentanoic acid and 2-piperidone from ...
1984-12-01 [J. Neurochem. 43(6) , 1631-4, (1984)] |
|
Palladium-catalyzed asymmetric 6-endo cyclization of dienami...
2012-05-04 [Org. Lett. 14(9) , 2326-9, (2012)] |
|
Synthesis and anticonvulsant activities of 3,3-dialkyl- and ...
1997-01-03 [J. Med. Chem. 40(1) , 44-9, (1997)] |
|
Synthesis and in vitro anthelmintic activity against Nippost...
1997-10-01 [Il Farmaco 52(10) , 603-8, (1997)] |