Small molecule inhibition of a Group II chaperonin: pinpointing a loop region within the equatorial domain as necessary for protein refolding.
Lisa M Bergeron, David L Shis, Lizabeth Gomez, Douglas S Clark
文献索引:Arch. Biochem. Biophys. 481(1) , 45-51, (2009)
全文:HTML全文
摘要
The functionality of regions within the equatorial domain of Group II chaperonins is poorly understood. Previously we showed that a 70 amino acid sequence within this domain on the single-subunit recombinant thermosome from Methanocaldococcus jannaschii (rTHS) contains residues directly responsible for refolding protein substrates [L.M. Bergeron, C. Lee, D.S. Clark, Identification of a critical chaperoning region on an archaeal recombinant thermosome, Biochem. Biophys. Res. Commun. 369 (2008) 707-711]. In the present study, 6-aminopenicillanic acid (6-APA) was found to bind to rTHS and inhibit it from refolding proteins. Fluorescence anisotropy was used to measure a 6-APA/rTHS dissociation constant of 17.1 microM and verify that the binding site is within the first 70 amino-terminal rTHS residues. Docking simulations point to a specific loop region at residues 53-57 on rTHS as the most likely binding region. This loop region is located within the oligomeric association sites of the wild-type thermosome. These results implicate a specific equatorial region of Group II chaperonins in the refolding of proteins, and suggest its importance in conformational changes that accompany chaperone function.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
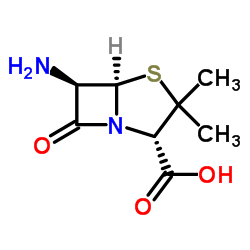 |
6-氨基青霉烷酸
CAS:551-16-6 |
C8H12N2O3S |
|
Enhanced production of 6-aminopenicillanic acid in aqueous m...
2010-01-01 [Prep Biochem Biotechnol. 40(1) , 38-45, (2010)] |
|
Monitoring bioreactors using principal component analysis: p...
2010-06-01 [Bioprocess Biosyst. Eng. 33(5) , 557-64, (2010)] |
|
Synthesis and biological evaluation of penem inhibitors of b...
2009-07-15 [Bioorg. Med. Chem. Lett. 19(14) , 3787-90, (2009)] |
|
FT-IR, FT-Raman, ab initio and DFT structural and vibrationa...
2010-01-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 75(1) , 183-90, (2010)] |
|
One-pot, two-step enzymatic synthesis of amoxicillin by comp...
2010-09-01 [Appl. Microbiol. Biotechnol. 88(1) , 49-55, (2010)] |