Synthesis of protoheme IX derivatives with a covalently linked proximal base and their human serum albumin hybrids as artificial hemoprotein.
Akito Nakagawa, Naomi Ohmichi, Teruyuki Komatsu, Eishun Tsuchida
文献索引:Org. Biomol. Chem. 2(21) , 3108-12, (2004)
全文:HTML全文
摘要
The simple one-pot reaction of protoporphyrin IX and omega-(N-imidazolyl)alkylamine or O-methyl-L-histidyl-glycine with benzotriazol-1-yl-oxytris(dimethylamino)phosphonium hexafluorophosphate at room temperature produced a series of protoporphyrin IX species with a covalently linked proximal base at the propionate side-chain. The central iron was inserted by the general FeCl2 method, converting the free-base porphyrins to the corresponding protoheme IX derivatives. Mesoporphyrin IX and diacetyldeuteroporphyrin IX analogues were also prepared by the same procedure. The Fe(II) complexes formed dioxygen (O2) adducts in dimethylformamide at 25 degrees C. Some of them were incorporated into the hydrophobic domain of recombinant human serum albumin (rHSA), providing albumin-heme hybrids (rHSA-heme), which can bind and release O2 in aqueous media (pH 7.3, 25 degrees C). The oxidation process of converting the dioxygenated heme in rHSA to the inactive Fe(III) state obeyed first-order kinetics, indicating that the mu-oxo dimer formation was prevented by the immobilization of heme in the albumin scaffold. The rHSA-heme, in which the histidylglycil tail coordinates to the Fe(II) center, showed the most stable O2 adduct complexes.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
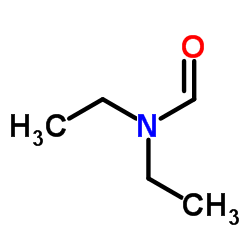 |
N,N-二乙基甲酰胺
CAS:617-84-5 |
C5H11NO |
|
Fabrication of porous cubic architecture of ZnO using Zn-ter...
2013-12-16 [Inorg. Chem. 52(24) , 14028-33, (2013)] |
|
A new and facile synthesis of quinazoline-2,4(1H,3H)-diones.
2009-03-19 [Org. Lett. 11(6) , 1193-6, (2009)] |
|
Metal-catalyzed reduction of HCONR'2, R' = Me (DMF), Et (DEF...
2012-01-18 [J. Am. Chem. Soc. 134(2) , 848-51, (2012)] |
|
Glass-forming tendency in the system water-dimethyl sulfoxid...
2000-03-01 [Cryobiology 40(2) , 151-8, (2000)] |
|
Oxidation of N,N-dimethylformamide and N,N-diethylformamide ...
2001-10-15 [Toxicol. Lett. 124(1-3) , 11-9, (2001)] |