The inactivation of bifunctional peptidylglycine alpha-amidating enzyme by benzylhydrazine: evidence that the two enzyme-bound copper atoms are nonequivalent.
D J Merkler, R Kulathila, D E Ash
文献索引:Arch. Biochem. Biophys. 317(1) , 93-102, (1995)
全文:HTML全文
摘要
Peptidylglycine alpha-amidating enzyme catalyzes the two-step conversion of C-terminal glycine-extended peptides to C-terminal alpha-amidated peptides and glyoxylate in a reaction that requires O2, ascorbate and 2 mol of copper per mole of enzyme [Kulathila et al. (1994) Arch. Biochem. Biophys. 311, 191-195]. Peptides with a C-terminal alpha-hydroxyglycine residue are intermediates in the amidation reaction. Benzylhydrazine inactivates the enzymatic conversion of dansyl-Tyr-Val-Gly to dansyl-Tyr-Val-NH2 in a time- and concentration-dependent manner. In contrast, the enzymatic conversion of dansyl-Tyr-Val-alpha-hydroxyglycine to dansyl-Tyr-Val-NH2 is unaffected by benzylhydrazine. The plot of 1/(inactivation rate) vs 1/[benzylhydrazine] is parabolic, indicating that the inactivation results from the interaction of 2 mol of benzylhydrazine per mole of enzyme. EPR spectra obtained from benzylhydrazine inactivation reactions carried out in the presence of a radical trap, alpha-(4-pyridyl-1-oxide)-N-tert-butylnitrone, show the formation of a carbon-centered benzyl radical. The benzyl radical most likely results from redox chemistry between benzylhydrazine and the enzyme-bound Cu(II) ions because EPR studies show that enzyme-bound Cu(II) is reduced to Cu(I) in the presence of benzylhydrazine. The kinetic constants for benzylhydrazine as a reductant in the amidation reaction were determined at benzylhydrazine concentrations too low to cause significant enzyme inactivation. Mimosine exhibits mixed inhibition vs benzylhydrazine; however, previous results have shown that benzylhydrazine is competitive vs ascorbate [Miller et al. (1992) Arch. Biochem. Biophys. 298, 380-388]. This change in kinetic mechanism coupled with the nonlinear inactivation kinetics have lead to a proposal that the two enzyme-bound Cu(II) atoms are nonequivalent with respect to their reduction by benzylhydrazine.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
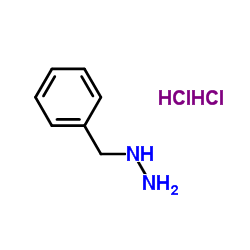 |
苄基肼二盐酸盐
CAS:20570-96-1 |
C7H12Cl2N2 |
|
Determination of hydrazine in pharmaceuticals IV: Hydrazine ...
1985-01-01 [J. Pharm. Sci. 74(1) , 105-7, (1985)] |
|
Benzylhydrazine as a pseudo-substrate of bovine serum amine ...
1989-05-15 [Biochem. J. 260(1) , 19-25, (1989)] |
|
Discovery and SAR of indole-2-carboxylic acid benzylidene-hy...
2004-07-01 [Bioorg. Med. Chem. 12(13) , 3649-55, (2004)] |
|
Selective mechanism-based inactivation of peptidylglycine al...
1997-06-01 [Biochem. Pharmacol. 53(11) , 1695-702, (1997)] |
|
The role of copper in bovine serum amine oxidase.
1990-01-01 [Biol. Met. 3(2) , 114-7, (1990)] |