Immobilization of (1S,2R)-(+)-2-amino-1,2-diphenylethanol derivates on aminated silica gel with different linkages as chiral stationary phases and their enantioseparation evaluation by HPLC.
Chuan-Qi Yin, Bao-Jiang He, Shi-Rong Li, Yong-Qiong Liu, Zheng-Wu Bai
文献索引:Chirality 21(4) , 442-8, (2009)
全文:HTML全文
摘要
A chiral selector was prepared through the reaction between (1S,2R)-(+)-2-amino-1,2-diphenylethanol and phenyl isocyanate. This selector was immobilized on aminated silica gel, respectively, with bifunctional group linkers of 1,4-phenylene diisocyanate, methylene-di-p-phenyl diisocyanate, and terephthaloyl chloride to produce corresponding three chiral stationary phases. The prepared compounds and chiral stationary phases were characterized by FT-IR, elemental analysis, (1)H NMR, and solid-state (1)H NMR. The enantioseparation ability of these chiral stationary phases was evaluated with structurally various chiral compounds. The chiral stationary phase prepared with 1,4-phenylene diisocyanate as linker showed excellent enantioseparation ability. The influence of different linkages on the enantioseparation was discussed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
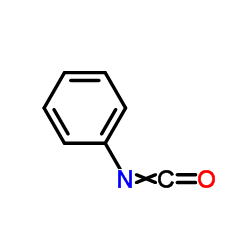 |
异氰酸苯酯
CAS:103-71-9 |
C7H5NO |
|
Ionic liquid-mediated technology to produce cellulose nanocr...
2015-12-10 [Carbohydr. Polym. 134 , 609-16, (2015)] |
|
Towards determination of absolute molar mass of cellulose po...
2015-08-28 [J. Chromatogr. A. 1409 , 53-9, (2015)] |
|
Chemically modified cellulose paper as a thin film microextr...
2013-11-01 [J. Chromatogr. A. 1314 , 24-30, (2013)] |
|
Design, synthesis and ¹H NMR study of C3v-symmetric anion re...
2016-01-15 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 153 , 199-205, (2015)] |
|
Analysis of protein adduction kinetics by quantitative mass ...
2007-06-30 [Chem. Biol. Interact. 168(2) , 117-27, (2007)] |