Charge transfer complexes of 9-vinyl-carbazole with pi acceptors in homogeneous and in micellar solutions.
Mauricio S Matos, Marcelo H Gehlen
文献索引:Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 60(7) , 1421-6, (2004)
全文:HTML全文
摘要
The molecular association of 9-vinyl-carbazole (CBZ) with three electron acceptors, p-chloranil (CHL), 2,7-dinitro-9-fluorenone (FL), and tetracyano-p-quinodimethane (TCNQ), is studied in acetonitrile and in micellar aqueous solution of sodium dodecyl sulfate (SDS). In both media, stable charge transfer (CT) complexes are formed with association constants in the range of 8-500 M(-1). CBZ and FL form a 1:2 complex in acetonitrile, but in SDS micelles the association is 1:1 due to size restriction and occupancy statistics in the host aggregates. The combination of absorption and fluorescence emission spectroscopy data indicates that the bimolecular CT complex of CBZ with TCNQ is stabilized in two distinct environments of the SDS micelles providing then two separated CT absorption bands.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
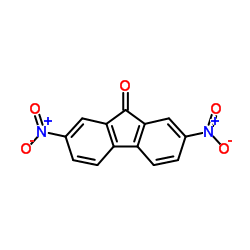 |
2,7-二硝基-9-芴
CAS:31551-45-8 |
C13H6N2O5 |
|
Spontaneous and mutagen-induced deletions: mechanistic studi...
1984-07-01 [Proc. Natl. Acad. Sci. U. S. A. 81(14) , 4457-61, (1984)] |
|
Mutagenicity of diesel-exhaust particle extract, 1-nitropyre...
1983-12-01 [Mutat. Res. 124(3-4) , 191-200, (1983)] |
|
Potent mutagenic impurities in a commercial sample of 3-nitr...
1982-01-01 [Cancer Lett. 15(3) , 209-14, (1982)] |
|
Voltammetric Determination of Genotoxic Nitro Derivatives of...
[Electroanalysis 22(17-18) , 2034-2042, (2010)] |
|
Preparation and characterization of a 1: 1 complex of tetrat...
[Synthetic Metals 58(1) , 73-76, (1993)] |