Thiamine biosynthesis in Escherichia coli: in vitro reconstitution of the thiazole synthase activity.
Roberta Leonardi, Peter L Roach
文献索引:J. Biol. Chem. 279(17) , 17054-62, (2004)
全文:HTML全文
摘要
The biosynthesis of thiamine in Escherichia coli requires the formation of an intermediate thiazole from tyrosine, 1-deoxy-d-xylulose-5-phosphate (Dxp), and cysteine using at least six structural proteins, ThiFSGH, IscS, and ThiI. We describe for the first time the reconstitution of thiazole synthase activity using cell-free extracts and proteins derived from adenosine-treated E. coli 83-1 cells. The addition of adenosine or adenine to growing cultures of Aerobacter aerogenes, Salmonella typhimurium, and E. coli has been shown previously to relieve the repression by thiamine of its own biosynthesis and increase the expression levels of the thiamine biosynthetic enzymes. By exploiting this effect, we show that the in vitro thiazole synthase activity of cleared lysates or desalted proteins from E. coli 83-1 cells is dependent upon the addition of purified ThiGH-His complex, tyrosine (but not cysteine or 1-deoxy-d-xylulose-5-phosphate), and an as yet unidentified intermediate present in the protein fraction from these cells. The activity is strongly stimulated by the addition of S-adenosylmethionine and NADPH.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
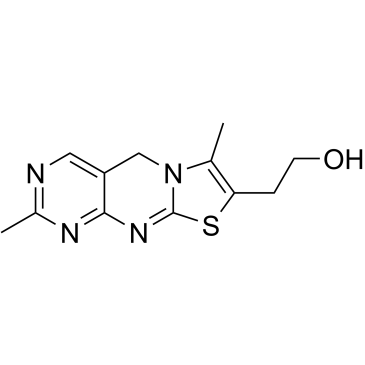 |
硫色素
CAS:92-35-3 |
C12H14N4OS |
|
Functional activation of G-proteins coupled with muscarinic ...
2014-01-01 [J. Pharmacol. Sci. 125(2) , 157-68, (2014)] |
|
Salvage of the thiamin pyrimidine moiety by plant TenA prote...
2014-10-01 [Biochem. J. 463(1) , 145-55, (2014)] |
|
Determination of thiamine and its phosphate esters in rat ti...
2005-02-25 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 816(1-2) , 67-72, (2005)] |
|
A reliable semiautomated method for the determination of tot...
1982-01-01 [Ann. Clin. Biochem. 19(Pt 1) , 52-6, (1982)] |
|
[Effect of thiochrome capronate on lipid metabolism in rats]...
1986-01-01 [Ukr. Biokhim. Zh. 58(5) , 89-91, (1986)] |