Glutathione monoethyl ester: high-performance liquid chromatographic analysis and direct preparation of the free base form.
E B Campbell, O W Griffith
文献索引:Anal. Biochem. 183 , 21, (1989)
全文:HTML全文
摘要
Glutathione monoethyl ester (L-gamma-glutamyl-L-cysteinylglycine ethyl ester) was shown by R. N. Puri and A. Meister (1983, Proc. Natl. Acad. Sci. USA 80, 5258-5260) to be taken up by several tissues and intracellularly hydrolyzed to GSH. Since GSH itself is not significantly taken up by tissues, glutathione monoesters provide the most direct and convenient means available for increasing the intracellular GSH concentration of many tissues and cell types. In previous studies glutathione esters were prepared by HCl- or H2SO4-catalyzed esterification, and the product esters were precipitated as acidic salts by addition of ether to the reaction mixtures. In the present studies, glutathione monoethyl ester was synthesized by H2SO4-catalyzed esterification in the presence of sodium sulfate as the dehydrating agent. When no GSH remained, alcohol-washed Dowex-1 resin (hydroxide form) was added to remove sulfate and neutralize the reaction mixture. After the resin was removed by filtration, glutathione monoethyl ester crystallized in the chilled filtrate. The product was free of sulfate, GSH, and glutathione diester; its solutions in water or saline were neutral. Preparations obtained to date are nontoxic when administered to mice in doses up to at least 10 mmol/kg. Progress of the esterification reaction and purity of the product were determined quantitatively by HPLC after derivatization of the thiols with monobromobimane. Elution times of GSH, glutathione diester, and glutathione monoesters involving either the glutamyl or the glycyl carboxylate groups are reported.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
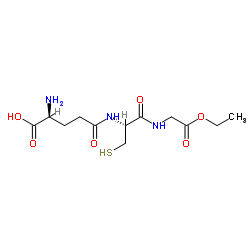 |
谷胱甘肽乙基酯
CAS:92614-59-0 |
C12H21N3O6S |
|
PRIMA-1Met induces myeloma cell death independent of p53 by ...
2014-09-04 [Blood 124(10) , 1626-36, (2014)] |
|
Dual-targeting of aberrant glucose metabolism in glioblastom...
2015-01-01 [J. Exp. Clin. Cancer Res 34 , 14, (2015)] |
|
Fibroblasts that resist cigarette smoke-induced senescence a...
2014-09-01 [Am. J. Physiol. Lung Cell. Mol. Physiol. 307(5) , L364-73, (2014)] |
|
Oxidant-mediated lung epithelial cell tolerance: the role of...
2001-09-15 [Biochem. Pharmacol. 62 , 787-794, (2001)] |
|
Glutathione monoethyl ester: preparation, uptake by tissues,...
1985-06-01 [Arch. Biochem. Biophys. 239 , 538, (1985)] |