Determination of trace cadmium in waters by flame atomic absorption spectrophotometry after preconcentration with 1-nitroso-2-naphthol-3,6-disulfonic acid on Ambersorb 572.
M Levy, R Safadi, E Zylber-Katz, L Granit, Y Caraco
文献索引:Ann. Chim. 95(1-2) , 77-85, (2005)
全文:HTML全文
摘要
A procedure for the determination of trace amount of cadmium after adsorption of its 1-nitroso-2-naphthol-3,6-disulfonic acid chelate on Ambersorb 572 has been proposed. This chelate is adsorbed on the adsorbent in the pH range 3-8 from large volumes of aqueous solution of water samples with a preconcentration factor of 200. After being sorbed, cadmium was eluted by 5 mL of 2.0 mol L(-1) nitric acid solution and determined directly by flame atomic absorption spectrophotometery (FAAS). The detection limit (3sigma) of cadmium was 0.32 microg L(-1). The precision of the proposed procedure, calculated as the relative standard deviation of recovery in sample solution (100 mL) containing 5 microg of cadmium was satisfactory (1.9%). The adsorption of cadmium onto adsorbent can formally be described by a Langmuir equation with a maximum adsorption capacity of 19.6 mg g(-1) and a binding constant of 6.5 x 10(-3) L mg(-1). Various parameters, such as the effect of pH and the interference of a number of metal ions on the determination of cadmium, have been studied in detail to optimize the conditions for the preconcentration and determination of cadmium in water samples. This procedure was applied to the determination of cadmium in tap and river water samples.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
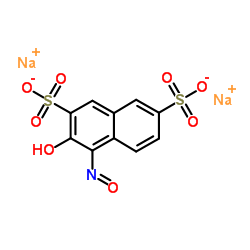 |
亚硝基红盐
CAS:525-05-3 |
C10H5NNa2O8S2 |
|
Cobalt skin dose resulting from short and repetitive contact...
2014-06-01 [Contact Dermatitis 70(6) , 361-8, (2014)] |
|
A spot test for detection of cobalt release - early experien...
2010-08-01 [Contact Dermatitis 63(2) , 63-9, (2010)] |
|
Metal ion binding to D-xylose isomerase from Streptomyces vi...
1988-02-15 [Biochem. J. 250(1) , 285-90, (1988)] |
|
Substoichiometric determination of cobalt in crud.
2015-05-07 [Radioisotopes 34(4) , 201-6, (1985)] |
|
Column chromatographic pre-concentration of iron(III) in all...
1990-09-01 [Analyst 115(9) , 1191-5, (1990)] |