Versatile access to benzhydryl-phenylureas through an unexpected rearrangement during microwave-enhanced synthesis of hydantoins.
Giulio G Muccioli, Johan Wouters, Jacques H Poupaert, Bernadette Norberg, Wolfgang Poppitz, Gerhard K E Scriba, Didier M Lambert
文献索引:Org. Lett. 5(20) , 3599-602, (2003)
全文:HTML全文
摘要
[reaction: see text] A new access to benzhydryl-phenylureas is described. These new interesting urea derivatives were obtained by reaction of substituted benzils with substituted phenylureas under microwave irradiation. Phenylthiourea, when reacted with benzil, gave 3-phenyl-thiohydantoin. Moreover, benzylurea, as phenethylurea, gave the corresponding 3-substituted hydantoin derivatives, demonstrating that only phenylurea derivatives can result in benzhydryl-phenylureas under the applied conditions. This new reaction proved to be an easy access to substituted 1-benzhydryl-3-phenyl-ureas.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
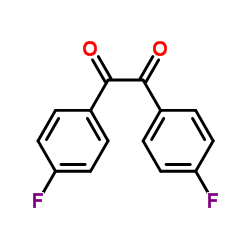 |
二氟苯偶酰
CAS:579-39-5 |
C14H8F2O2 |
|
Iridium(III) complexes with orthometalated quinoxaline ligan...
2005-03-07 [Inorg. Chem. 44(5) , 1344-53, (2005)] |
|
Studies on the Condensation Pathway to and Properties of Dii...
2009-03-01 [Organometallics 26(8) , 1907-1911, (2007)] |
|
Synthesis and investigation of the properties of aromatic po...
[Acta Polym. 42(2-3) , 82--86, (1991)] |
|
Yellow organic light-emitting diodes based on phosphorescent...
[Inorganica Chim. Acta 362(7) , 2231-26, (2009)] |
|
Synthesis and Characterization of N-(2-Ethylhexyl) carbazole...
[Bull. Korean Chem. Soc. 31(7) , 2073-76, (2010)] |