Use of N-methylformamide as a solvent in indium-promoted Barbier reactions en route to enediyne and epoxy diyne formation: comparison of rate and stereoselectivity in C-C bond-forming reactions with water.
Kwame Frimpong, Joseph Wzorek, Claire Lawlor, Katharine Spencer, Thomas Mitzel
文献索引:J. Org. Chem. 74(16) , 5861-70, (2009)
全文:HTML全文
摘要
Indium-promoted coupling reactions between propargyl aldehydes (1) and alpha-chloropropargylphenyl sulfide are reported. Although water has been shown to accelerate indium metal promoted reactions, the reverse pattern was observed in this series. Use of N-methylformamide (NMF), which has not previously been a solvent known for use in indium-promoted reactions, afforded an acceleration of these Barbier-style reactions compared to water. Indium-promoted reactions in this study also showed excellent regiocontrol and good stereocontrol, allowing for easy entry into the formation of epoxydiyne and enediyne skeletal structures. This paper also describes use of the Barbier Coupled product (2) as a new, and easy, entry into the formation of enediyne and epoxydiyne skeletal structures.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
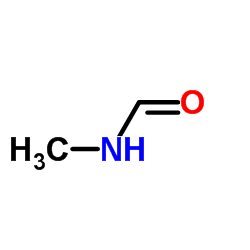 |
N-甲基甲酰胺
CAS:123-39-7 |
C2H5NO |
|
Surface-enhanced resonance Raman scattering nanostars for hi...
2015-01-21 [Sci. Transl. Med. 7(271) , 271ra7, (2015)] |
|
Mitochondrial DNA alterations in blood of the humans exposed...
2007-02-20 [Chem. Biol. Interact. 165(3) , 211-9, (2007)] |
|
Amidic and acetonic cryoprotectants improve cryopreservation...
2012-01-01 [Cryo. Letters 33(3) , 202-13, (2012)] |
|
N-methylformamide: antitumour activity and metabolism in mic...
1982-06-01 [Br. J. Cancer 45(6) , 843-50, (1982)] |
|
Theoretical exploration of the cooperative effect in NMF-NMF...
2008-09-28 [Phys. Chem. Chem. Phys. 10(36) , 5607-15, (2008)] |