Inhibitors of bacterial cystathionine beta-lyase: leads for new antimicrobial agents and probes of enzyme structure and function.
Linda J Ejim, Jan E Blanchard, Kalinka P Koteva, Rachael Sumerfield, Nadine H Elowe, Jonathan D Chechetto, Eric D Brown, Murray S Junop, Gerard D Wright
文献索引:J. Med. Chem. 50 , 755-64, (2007)
全文:HTML全文
摘要
The biosynthesis of methionine is an attractive antibiotic target given its importance in protein and DNA metabolism and its absence in mammals. We have performed a high-throughput screen of the methionine biosynthesis enzyme cystathionine beta-lyase (CBL) against a library of 50 000 small molecules and have identified several compounds that inhibit CBL enzyme activity in vitro. These hit molecules were of two classes: those that blocked CBL activity with mixed steady-state inhibition and those that covalently interacted with the enzyme at the active site pyridoxal phosphate cofactor with slow-binding inhibition kinetics. We determined the crystal structure of one of the slow-binding inhibitors in complex with CBL and used this structure as a guide in the synthesis of a small, focused library of analogues, some of which had improved enzyme inhibition properties. These studies provide the first lead molecules for antimicrobial agents that target cystathionine beta-lyase in methionine biosynthesis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
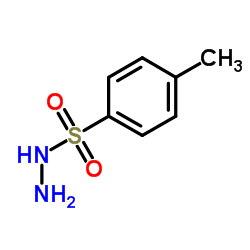 |
对甲苯磺酰肼
CAS:1576-35-8 |
C7H10N2O2S |
|
Protecting group free glycosidations using p-toluenesulfonoh...
2008-08-21 [Org. Lett. 10(16) , 3461-3, (2008)] |
|
Synthesis of some p-toluenesulfonyl-hydrazinothiazoles and h...
2010-11-01 [Eur. J. Med. Chem. 45(11) , 5080-5, (2010)] |
|
Vasoconstrictor-induced changes in renal blood flow: role of...
1988-04-01 [Am. J. Physiol. 254(4 Pt 2) , F470-6, (1988)] |
|
Mutagenic activity of p-toluenesulfonhydrazide.
1992-01-01 [Toxicol. Ind. Health 8(6) , 369-76, (1992)] |
|
Enantioselective synthesis of trifluoromethyl-substituted cy...
2007-07-05 [Org. Lett. 9 , 2625, (2007)] |